Abstract
Objective
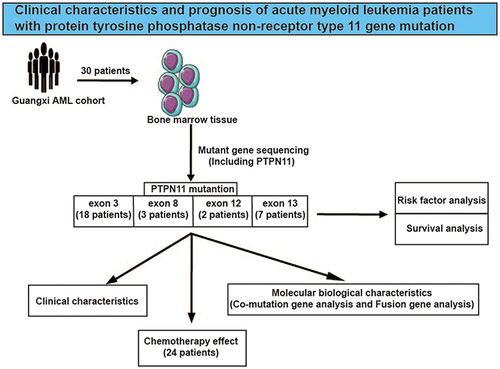
The purpose of our study was to investigate the clinical characteristics, molecular biological characteristics and prognosis of acute myeloid leukemia (AML) patients with protein tyrosine phosphatase non-receptor type 11 (PTPN11) gene mutation.
Methods
The clinical data of 30 newly diagnosed adult AML patients with PTPN11 gene mutation were analyzed retrospectively. Kaplan-Meier and Cox proportional risk regression model were examined for prognostic analysis and prognostic factor screening.
Results
High-frequency mutation sites of PTPN11 gene are located in exon 3 of chromosome 12, which are D61 and A72 (16.7%), followed by E76 (13.3%). The median variant allele frequency (VAF) of PTPN11 mutant gene is 18.4%. The patients were divided into two groups according to PTPN11 VAF 35.3% (upper quartile). We observed that the peripheral blood leukocyte count in patients with VAF ≥35.3% was significantly higher than patients with VAF < 35.3% (p = 0.019) and also closely related to M5 (p = 0.016) and internal tandem duplication (ITD) of FMS-like tyrosine kinase 3 (FLT3) (FLT3-ITD) mutation (p = 0.048). Taking PTPN11 VAF 20% and 35.3% as the cutoff value, the patients were divided into two groups, and the overall survival and event-free survival (EFS) of the two groups were not significant. Multivariate analysis of Cox risk ratio model showed that white blood cell count and Eastern Cooperative Oncology Group (ECOG) physical status score were independent risk factors affecting the EFS.
Conclusion
Our study observed that PTPN11 VAF may not be a prognostic factor in patients with PTPN11mut AML. Newly diagnosed high white blood cell count and poor performance status were independent risk factors for EFS in PTPN11mut AML.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2023-E232-01). The signed informed consent was obtained from all participants. This study complies with the Declaration of Helsinki.
Acknowledgments
We would also acknowledge the support by Key Laboratory of Hematology, Guangxi Medical University, Education Department of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Rui Huang and Yi-Ting Zhang are co-first authors for this study.
Disclosure
The authors declare no conflicts of interest in this work.