Figures & data
Table I. Phase III studies: baseline characteristics.
Figure 1. Mean “average pain over the past 24 hours” at week 12 of the 12-week RCT is shown, using the PMM and two sensitivity statistical analysis models (MAR and hybrid LOCF/BOCF).Abbreviations: LSM = Least squares mean; SE = Standard error; PMM = Pattern-mixture model; MAR = Missing at random model; BOCF = Baseline observation carried forward; LOCF = Last observation carried forward; RCT = Randomized controlled trial.
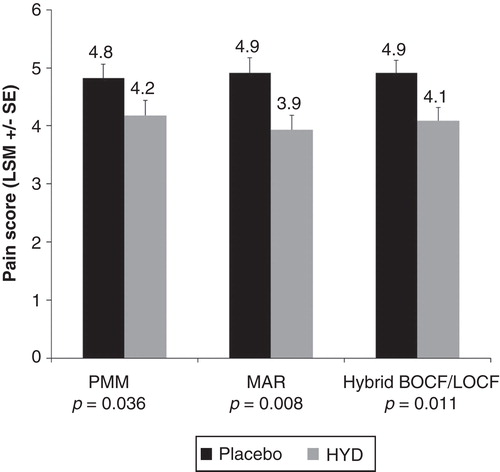
Figure 2. Mean weekly “average pain over the past 24 hours” during the open-label trial is shown.Abbreviations: SE = Standard error; S = Screening; TE = Titration end.
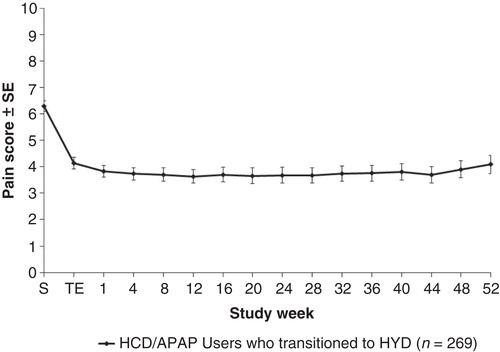
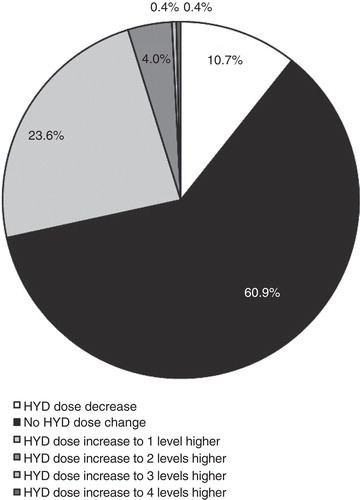
Figure 3. Mean doses of HYD (A) and non-trial opioid analgesics (B) during the open-label trial are shown.Abbreviations: SE = Standard error; S = Screening; TS = Titration start; TE = Titration end.
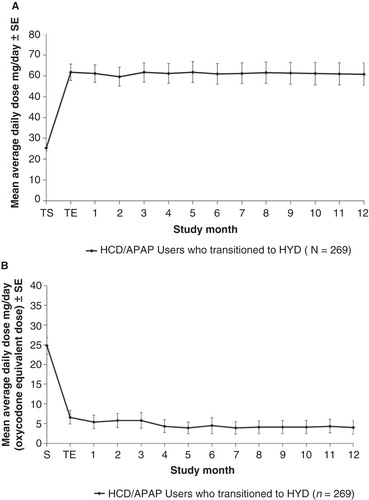
Table II. Summary of treatment satisfaction questionnaire in the open-label trial with 1-year maintenance treatment.
Table III. Phase III trials: adverse eventsa.