Figures & data
Figure 1. Studies Included in Safety Analyses.
APAP: acetaminophen (paracetamol)a Number of patients treated with at least one dose of BTDS. bSeven of the 14 controlled studies had open-label extension periods. cN = 902 represents the number of patients treated with BTDS. Patients in the forced titration studies not randomized to BTDS may not have received BTDS; 198 patients who were randomized to placebo or active comparators in the double-blind period entered the open-label extension period and received BTDS. This open label extension study consisted of 377 patients from three of the six nonenriched, controlled studies plus eight additional patients without prior exposure to double-blind treatment in a previous study (385 total).
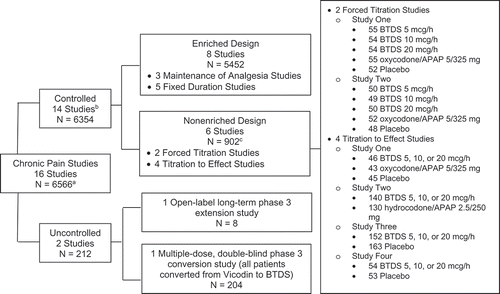
Table 1. Demographic and baseline characteristics by age for patients enrolled in 16 clinical trials of BTDS.
Table 2. Treatment exposure by age for patients enrolled in 16 clinical trials of BTDS.
Table 3. Medical history in 10% or greater of BTDS-treated patients by age (all study pool).
Table 4. Concomitant medications taken by ≥25% of patients during BTDS exposure by age (all study pool).
Table 5. Incidence of adverse events (AEs) in ≥2% of patients by age group enrolled in 16 clinical trials of BTDS.
Table 6. Incidence of AEs with treatment-by-age interaction for patients enrolled in 16 clinical trials of BTDS.
Table 7. Incidence of AEs in the nonenriched, controlled study pool with treatment-by-age interaction.
Table 8. Odds ratios and 95% confidence intervals for AEs with no statistically significant treatment-by-age interaction in the nonenriched, controlled study pool (adjusted analyses).
Table 9. Shifts in ALT and AST values during BTDS exposure (all study pool).
