Figures & data
Figure 1. Ultrasound backscatter image monitoring of a 3100 W cm−2 (free field spatial peak intensity) exposure of excised bovine liver imaged with a Z.One scanner (Zonare Medical Systems Inc., USA) using an 8-MHz linear probe. The FUS beam was incident from the left. Top images are B-scan images obtained with a frame rate of 14 Hz. Middle is a photograph of the lesion. Bottom are subtraction images: left is the result of subtracting the top left B scan from the top right; right is a similar result using a B scan image obtained 3.6 s later. The hyperechogenicity is due to bubbles, probably from boiling, and the reduction with time is due to cooling. Courtesy of Jim McLauglan, Institute of Cancer Research.
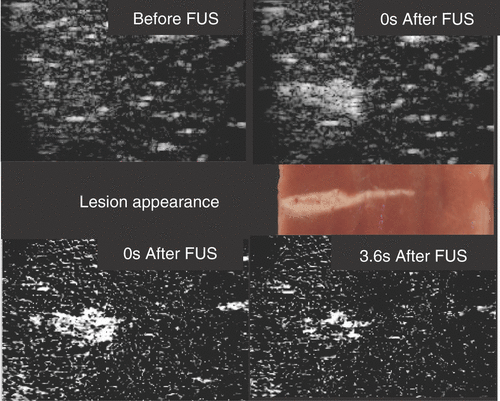
Figure 2. This collage depicts three different types of thermocouple: (left) thin-film thermocouple (NPL, UK), (centre) soldered fine-wire thermocouple (ICR, UK), and (right) hypodermic needle thermocouples (Omega, USA).
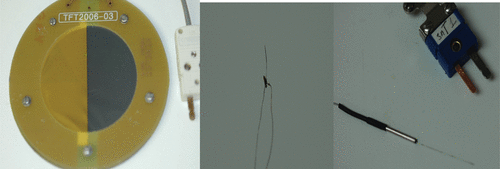
Figure 3. Comparison of the temperature rise in excised bovine liver during a 150-W cm−2 (free field spatial peak intensity), 5-s exposure measured with a fine-wire thermocouple (FWT) and a thin-film thermocouple (TFT). The FWT suffers from viscous heating artefact whereas the TFT does not; therefore, the subtracted data (FWT-TFT) indicates viscous heating.
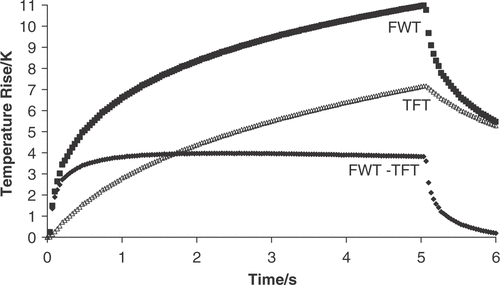
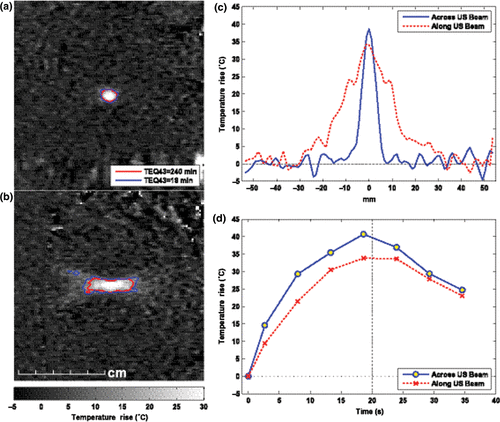
Figure 4. MRI-based temperature mapping during focused ultrasound thermal ablation of a uterine fibroid using the Exablate 2000 device (InSightec, Haifa, Israel). (a)–(b) Temperature maps acquired at peak temperature rise during two 20-s exposures. Contours show regions that reached thermal dose values of at least 18 and 240 equivalent minutes at 43°C. Based on previous in vivo work, the edge of thermally induced lesions should lie between these contours, yielding a lesion that is approximately 0.8 cm wide and 2.8 cm long. In practice, the ablated volume within the fibroid is often increased, presumably due to vascular occlusion. (a) Coronal imaging in the focal plane. (b) Sagittal imaging along ultrasound beam direction. (c) Spatial temperature distribution at peak temperature rise. (d) Temperature rise vs. time at the focus. (Figure courtesy of Nathan McDannold, PhD, Harvard Medical School, Brigham & Women's Hospital Department of Radiology, Boston, MA, USA.)