Figures & data
Figure 1. (a) The HT2000 infrared radiant heat device being closed. (b) HT2000 infrared radiant heat device with heating lights off, and soft tent sides folded over patient.
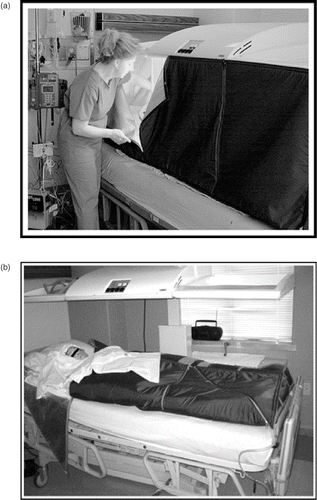
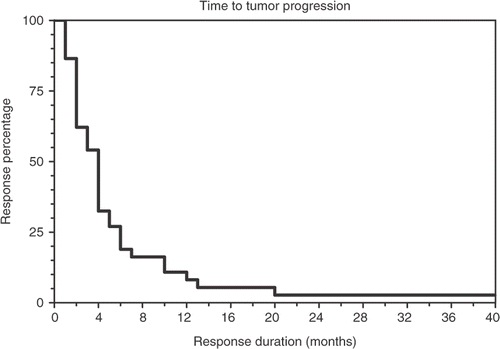
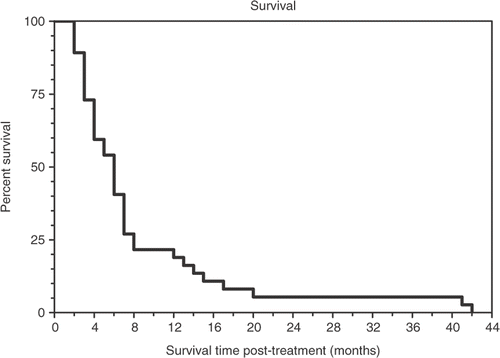
Table I. Tumor diagnosis of individual patients, age, sex, race, stage, number of treatment cycles, response, time to tumor progression (duration in months), and prior therapy.
Table II. Toxicities per cisplatin dose: Hematological and non-hematological.
Table III. Tumor response by cisplatin dose (40–70 mg/m2) evaluated using response evaluation criteria in solid tumors (RECIST).