Figures & data
Figure 1. Composition of CRCL prepared from human melanoma cell lines. (a) 15 mg of total protein from A375-MEL cell lysate were loaded onto a Rotofor device and FS-IEF was performed. Twenty fractions (each 2.5 ml) were harvested and aliquots of 15 µl were analysed by SDS-PAGE and Western blot for human Hsp90, Hsp70/Hsc70, Grp94/gp96 and calreticulin, using specific antibodies. Chaperones were detected in fractions 9-13, spanning a pH range from 5.4–6.5. (b) Fractions 9–13 of eight to ten independent Rotofor runs were combined to yield the CRCL. CRCL and cell lysates were analysed by SDS-PAGE and subsequent Western blot. Equal protein amounts of lysates and CRCL preparations, respectively, were loaded. Membranes stained with Ponceau S show the overall protein patterns of CRCL and cell lysates from melanoma cell lines A375-MEL and 624.38-MEL. (c) Membranes were developed using antibodies to chaperones, Hsp70/Hsc70, Hsp90, Grp94/gp96, calreticulin, and melanoma-associated antigens, tyrosinase and Melan-A/MART-1.
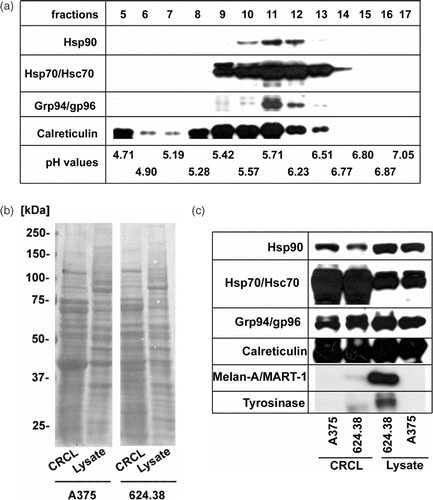
Figure 2. CRCL enhance cross-presentation of exogenous peptides, with heat-induced CRCL showing superior activity. (a) CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS) or CRCL(624.38-HS) respectively) (90 µg/ml) were mixed with the hybrid peptide pep70-Tyr (10 µM) and incubated for 2 h at RT. 1.5 × 104 DC were added for 1 h at 37°C/5% CO2 followed by the addition of 4 × 103 TyrF8 CTL. After 24 h IFN-γ in the supernatants was determined by ELISA. DC/CTL co-cultures with pep70-Tyr alone, with CRCL alone and without pep70-Tyr/CRCL served as controls. The relative IFN-γ production was calculated using IFN-γ of DC/CTL co-cultures with pep70-Tyr alone as reference value Citation[16]. Bars are the mean ± SD of three different experiments each performed in triplicate values. All cultures containing CRCL resulted in significantly higher CTL stimulation compared to controls with peptide alone (all p-values ≤0.05). (b) Chaperone and antigen levels of melanoma cells after heat treatment. 624.38-MEL cells were treated with sublethal heat shock (HS) (41.8°C for 2h) and allowed to recover for 20 h. Viable cells were harvested and analysed by Western blot for Hsp70, Hsc70, tyrosinase and Melan-1/MART-1. ß-actin served as loading control. (c) Chaperone content of CRCL derived from heat-treated melanoma cells. Equal amounts (10 µg) of CRCL(624.38 + HS) or CRCL(624.38 − HS) were analysed for Hsp90, Hsp70, Hsc70, Grp94/gp96 and calreticulin.
![Figure 2. CRCL enhance cross-presentation of exogenous peptides, with heat-induced CRCL showing superior activity. (a) CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS) or CRCL(624.38-HS) respectively) (90 µg/ml) were mixed with the hybrid peptide pep70-Tyr (10 µM) and incubated for 2 h at RT. 1.5 × 104 DC were added for 1 h at 37°C/5% CO2 followed by the addition of 4 × 103 TyrF8 CTL. After 24 h IFN-γ in the supernatants was determined by ELISA. DC/CTL co-cultures with pep70-Tyr alone, with CRCL alone and without pep70-Tyr/CRCL served as controls. The relative IFN-γ production was calculated using IFN-γ of DC/CTL co-cultures with pep70-Tyr alone as reference value Citation[16]. Bars are the mean ± SD of three different experiments each performed in triplicate values. All cultures containing CRCL resulted in significantly higher CTL stimulation compared to controls with peptide alone (all p-values ≤0.05). (b) Chaperone and antigen levels of melanoma cells after heat treatment. 624.38-MEL cells were treated with sublethal heat shock (HS) (41.8°C for 2h) and allowed to recover for 20 h. Viable cells were harvested and analysed by Western blot for Hsp70, Hsc70, tyrosinase and Melan-1/MART-1. ß-actin served as loading control. (c) Chaperone content of CRCL derived from heat-treated melanoma cells. Equal amounts (10 µg) of CRCL(624.38 + HS) or CRCL(624.38 − HS) were analysed for Hsp90, Hsp70, Hsc70, Grp94/gp96 and calreticulin.](/cms/asset/7663e2d1-139a-4a2b-bb92-5afbda1014c3/ihyt_a_321505_f0002_b.gif)
Figure 3. Melanoma-derived CRCL do not transfer tyrosinase or Melan-A/MART-1 antigens endogenously expressed by melanoma cells to human DC for MHC class I-restricted CTL recognition. (a, b) Monocyte-derived i-DC were pulsed with titered amounts of CRCL from A375-MEL (antigen-negative) or 624.38-MEL (antigen-positive) for 16 h and then matured with a cocktail containing IL1-ß, IL-6, TNF-α and PGE2 for additional 24 h. Antigen-specific CTL (TyrF8 or A42-MART-1) were added to DC at a DC : T cell ratio of 1 : 1. After 24 h, supernatants were harvested and IFN-γ levels were analysed by ELISA. (c) To control the DC capacity for CTL activation and to detect inhibitory effects of CRCL, 10 µg/ml of tyrosinase or Melan-A/MART-1 peptides (Tyr368–376 or Melan-A/MART-126–35, respectively) were added to DC with or without A375-MEL-derived CRCL (250 µg/ml). One representative experiment of five is shown for each CTL (a and b). Controls (c) are shown exemplarily for TyrF8 CTL. SD are derived from triplicate values.
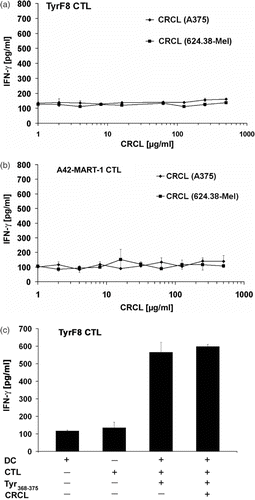
Figure 4. Inability to cross-present endogenous melanoma-associated antigens is independent of endogenous levels of Hsp70 or tyrosinase. (a) CRCL from Hsp70-enriched melanoma cells fail to cross-present endogenous tyrosinase. Monocyte-derived i-DC were incubated with titered concentrations of CRCL from heat-treated 624.38-MEL or melanoma cells cultured at 37°C (CRCL(624.38 + HS), CRCL(624.38 − HS), or CRCL(A375), respectively) for 2 h. A42-MART-1 CTL were added to yield a DC to T cell ratio of 1 : 1. After 24 h supernatants were harvested and IFN-γ levels were determined by ELISA. One of three representative experiments is shown. SD derived from triplicates. (b) Tyrosinase-overexpressing A375 melanoma cells. A375-MEL cells were infected with MVA-hTyr (Modified Vaccinia Virus Ankara containing the cDNA of the human tyrosinase gene) using a MOI of 5 U/ml. Cells were harvested 18 h after infection and analysed for human tyrosinase by SDS-PAGE and Western blot in parallel with uninfected A375-MEL and 624.38-MEL cells, which express the tyrosinase endogenously. ß-actin and Hsp70/Hsc70 served as loading control. (c) CRCL from tyrosinase-overexpressing melanoma cells do not facilitate cross-presentation of endogenous tyrosinase. Monocyte-derived i-DC were pulsed with titrated amounts of CRCL(A375), CRCL(624.38) or CRCL(A375/MVA-hTyr) for 6 h. DC were then matured with a cocktail containing IL1-ß, IL-6, TNF-α and PGE2 for 18 h. TyrF8 CTL were added at a DC to T cell ratio of 1 : 1. After 24 h, supernatants were harvested and IFN-γ levels were determined by ELISA. One of three representative experiments is shown. SD derived from triplicates. (d) Indicated amounts of recombinant Hsp70 protein were mixed to 20 µg/ml CRCL(A375/MVA-hTyr) and co-incubated with DC for 2 h followed by the addition of tyrosinase-specific CTL TyrF8. After 24 h supernatants were harvested for IFN-γ measurements. Control incubation of DC with tyrosinase nonamer peptide (Tyr368-376) at 1, 5 and 50 µM yielded IFN-γ values of 70, 114 and 250 pg/ml attesting that the DC presentation and CTL response was functional. All values are derived from triplicate cultures.
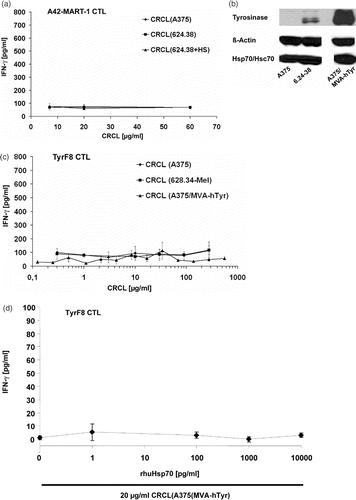
Figure 5. Human melanoma-derived CRCL do not change IL-12 secretion, MLR activity and uptake characteristics of i-DC. (a) Monocyte-derived i-DC (d7) were incubated with 40 µg/ml CRCL(624.38) for 24 h (uptake assay, MLR) or for 48 h (IL-12 production). Untreated and LPS (1 µg/ml)-treated cells served as controls. The IL-12p40-content of DC culture supernatants was determined by ELISA. One representative experiment of five is shown. (b) For MLR, untreated and CRCL-treated i-DC were harvested, irradiated (40 Gy) and co-cultured with allogeneic T cells at indicated ratios for 6 days. T cell proliferation was assessed by measuring the uptake of [3H]-thymidine (1 µCi/well [0.037 MBq]) during the last 24 h of culture. The values represent the mean of triplicate samples ± SD of one experiment representative of three. (c) For uptake assays, DC were harvested 24 h after treatment. Macropinocytosis was assessed using FITC-conjugated albumin at a final concentration of 0.5 mg/ml for 30 min. (d) FITC-conjugated Dextran at a final concentration of 10 µg/ml for 3.5 h was used to determine endocytosis. DC were analysed by flow cytometry. The bars represent the difference in MFI of FITC-Albumin or FITC-Dextran (FL1) obtained by subtracting the background MFI of cells incubated at 0°C from the MFI of cells incubated at 37°C. One representative experiment of three is shown respectively (c, d).
![Figure 5. Human melanoma-derived CRCL do not change IL-12 secretion, MLR activity and uptake characteristics of i-DC. (a) Monocyte-derived i-DC (d7) were incubated with 40 µg/ml CRCL(624.38) for 24 h (uptake assay, MLR) or for 48 h (IL-12 production). Untreated and LPS (1 µg/ml)-treated cells served as controls. The IL-12p40-content of DC culture supernatants was determined by ELISA. One representative experiment of five is shown. (b) For MLR, untreated and CRCL-treated i-DC were harvested, irradiated (40 Gy) and co-cultured with allogeneic T cells at indicated ratios for 6 days. T cell proliferation was assessed by measuring the uptake of [3H]-thymidine (1 µCi/well [0.037 MBq]) during the last 24 h of culture. The values represent the mean of triplicate samples ± SD of one experiment representative of three. (c) For uptake assays, DC were harvested 24 h after treatment. Macropinocytosis was assessed using FITC-conjugated albumin at a final concentration of 0.5 mg/ml for 30 min. (d) FITC-conjugated Dextran at a final concentration of 10 µg/ml for 3.5 h was used to determine endocytosis. DC were analysed by flow cytometry. The bars represent the difference in MFI of FITC-Albumin or FITC-Dextran (FL1) obtained by subtracting the background MFI of cells incubated at 0°C from the MFI of cells incubated at 37°C. One representative experiment of three is shown respectively (c, d).](/cms/asset/6d3e3498-452e-4675-91e8-f9679b6f4ac5/ihyt_a_321505_f0005_b.gif)
Table I. Human melanoma-derived CRCL do not change phenotypic markers of i-DC.