Figures & data
Figure 1. (A) Appearance of BR-TRG-I urinary bladder hyperthermia treatment system. (B) The schematic diagram of the pipeline system of BR-TRG-I urinary bladder hyperthermia treatment system. Figures adapted with permission from Qifei Li, Guangzhou Bright Medical Technology Co., Ltd.
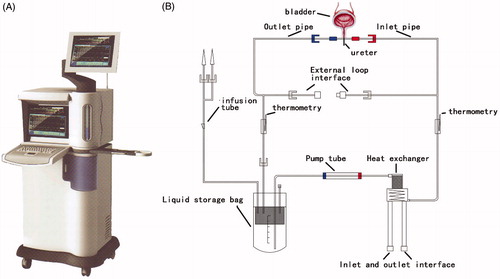
Figure 2. The schematic diagram of treatment of CTHC and THP groups. Above the dotted line is the induction treatments, maintenance treatments are below the dotted line. CTHC: three consecutive hyperthermia treatments combined with single instillations; HT: hyperthermia; THP: pirarubicin.
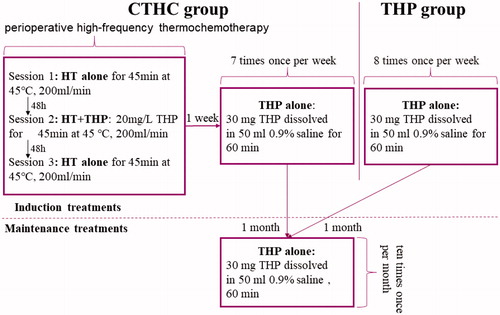
Figure 3. Diagram of this study. BCG: Bacillus Calmette-Guérin; Cis: carcinoma in situ; CTHC: three consecutive hyperthermia treatments combined with single instillations; MIBC: muscle invasive bladder cancer; NMIBC: nonmuscle invasive bladder cancer; MMC: mitomycin C; ITT: intention to treat; RC: radical cystectomy; TURBT: transurethral resection of bladder tumors; THP: pirarubicin.
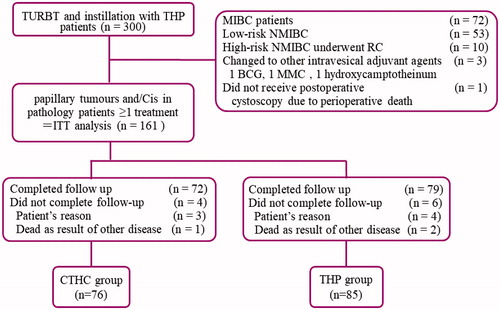
Table 1. Demographics and baseline characteristics (n = 161).
Figure 4. Bladder recurrence-free survival rates (A) and progression-free survival rates (B) of CTHC group and THP group estimated using the Kaplan–Meier method. The log-rank test was used for comparing recurrence-free survival rates and progression-free survival rates between the two groups. CTHC: three consecutive hyperthermia treatments combined with single instillations; THP: pirarubicin; TURBT: transurethral resection of bladder tumors.
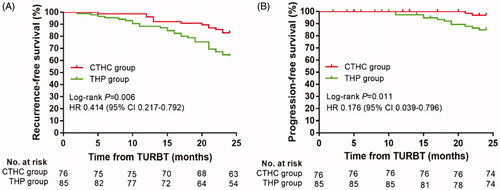
Table 2. Univariate and multivariate analysis of associated with recurrence.
Table 3. Comparison of adverse events between the two groups.
