Figures & data
Figure 1. All types of blood cells influence NSCLC progression through multiple mechanisms. At the primary tumor site (left part), activated Th1 cells secreting IFN-γ, IL-2 and TNF-β have strong antitumor activity and immunomodulatory effects, while Th2 cells secreting IL-4 and IL-10 can inhibit the response of Th1 cytokines, promoting tumor growth (Initiation). Activated neutrophils act as carcinogens through ROS, RNS and NE promoting tumorigenesis. It secretes IL-6, IL-8 and TNF-α that help CD8+ T cell to damage pre-tumoral cells and kill the tumor cells. Mature DCs can activate the initial T cells, killing and inhibiting tumor cells. Immature or semi-mature tumor-infiltrating DCs can participate in tumor immune escape, resulting in T cell anergy. Tumor cells can secrete IL-10 to interfere with the maturation of DCs. Erythrocytes can adhere to tumor cells and transfer to macrophages and NK cells. Moreover, peroxidase on the surface of erythrocytes can destroy the cell membrane of tumor cells at the adhesion spot. As the tumor progresses, neutrophils secrete NE activating tumor proliferation, arginase-1 suppressing CD8+ T cell and NK cell responses. Treg cells can inhibit the activation and proliferation of CD4+ and CD8 + T cells and kill effector cells. Alternatively activated macrophages (M2) inducing factors such as TGF-β and IL4 can inhibit tumor immunity. B cells can regulate the response of Treg cells and regulate T cells by expressing CD39 and CD73. Eosinophils secrete IL-4 and GM-CSF, strengthening the function of T cells and macrophages. Classically activated macrophages (M1) induce factors such as LPS and IFN-γ promoting tumor immunity. As the tumor grows, neutrophils release IL-1β, MMP-9 and VEGF to induce tumor angiogenesis and metastasis cooperating with leukotrienes (LT). Th17 cells, specifically secreting IL-17, promote tumor angiogenesis and metastasis. Eos secreted IL-12 inhibits the metastasis and infiltration of tumor cells. Activated platelets can increase angiogenesis in tumor tissues. Erythrocytes can adhere to circulating tumor cells(CTC) and carry them to macrophages and NK cells to prevent tumor cells from spreading and metastasizing through the blood. Platelet aggregation can promote the adhesion and encapsulation of CTC, assisting tumor cells to escape immune attack. Neutrophils can gather in the lung earlier than tumor cells to form early metastasis (right box). Neutrophils can suppress T cells and NK cells. Platelets can protect CTC from physical clearance and immune surveillance by mononuclear phagocyte system at the metastasis site.
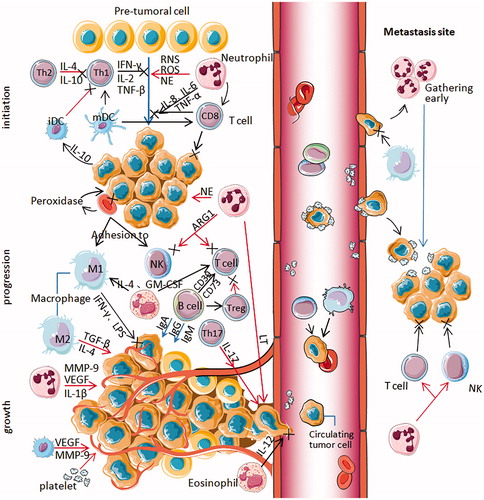
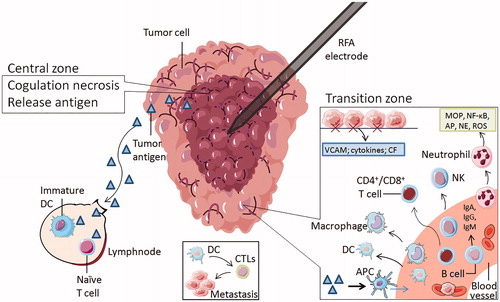
Figure 2. The zones of radiofrequency ablation to NSCLC. The RFA electrode is surrounded by two main zones. The central zone was coagulative necrosis at temperatures ≥50 °C, releasing intracellular antigens and inhibitors. Part of tumor antigen is drained to nearby lymph nodes, where it can stimulate immature DCs and naive T cells. Others are captured by antigen-presenting cells (APC), which can attract immune cells from the blood system. The transition zone at temperatures between 41 °C and 45 °C is still a heat-induced injury, but it is sublethal and reversible. The damaged local tissue exposes hyaluronic acid and endothelial injury markers, stimulate the expression of vascular adhesion molecules (VCAM), cytokines and chemotactic factor(CF), attract immune cells to this region and enhance some immune cells' antitumor function. These immune cells consist of neutrophils, B cells, macrophages, natural killer cells, dendritic cells, and CD4 + T and CD8 + T lymphocytes. Tumor cells are captured by erythrocytes and swallowed by APC. DC can induce CTLs to kill metastatic cells.