Figures & data
Figure 1. Tumor microenvironments are immunosuppressive which protects tumor cells and transforms immune cells from immunostimulatory to immunosuppressive. Left panel: tumor cells attract and recruit immune cells, such as macrophages, dendritic cells, and T cells. Right panel: however, the immunosuppressive nature of TME quickly suppress the activity of CD8 T-cells through multiple mechanisms mediated by MDSCs, tolerogenic DCs, M2-type macrophages and Treg cells. DC: dendritic cell; tol: tolerogenic; IDO: indoleamine 2,3-dioxygenase; Arg1: arginase; MDSC: myeloid-derived suppressor cells; NOX-2: nitric oxide synthase-2; PD(L)1: programmed death (ligand)-1; CTLA4: cytotoxic T lymphocyte-associated antigen; NO: nitric oxide; Treg: regulatory T-cell; IL: interleukin; TGF-β: transforming growth factor-β.
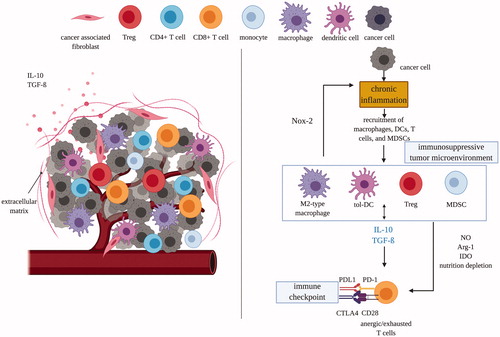
Figure 2. In situ vaccination can modulate tumor microenvironments to induce anti-tumor immune responses at multiple levels in the cancer immunity cycle. (A) ISV with immune adjuvant can cause local tissue damage, inducing immunogenic cell death and release of cancer cell antigens; and/or directly introduce PAMPs and/or DAMPs with TLR agonists or NPs into the tumor to (B) attract and activate dendritic cells and other antigen-presenting cells, (C) which mature and migrate from primary tumor to stimulate the priming and activation process of T cells in the tumor-draining lymph nodes. (D) Once primed and activated, T cells will then traffick to and (E) infiltrate solid tumors and metastases to (F) directly kill the tumors with granzymes and perforins. Multiple ISV strategies can be combined to achieve stronger anti-tumor immune response. ICD: immunogenic cell death. TAAs: tumor associated antigens. Immune adjuvants: injection of immune stimulating reagents. DAMPs: danger-associated molecular pattern molecules. PAMPs: pathogen associated molecular pattern molecules. HIFU: high intensity focused ultrasound. TLR: toll like receptor. NP: nanoparticles. Adapted from Chen and Mellman [Citation52].
![Figure 2. In situ vaccination can modulate tumor microenvironments to induce anti-tumor immune responses at multiple levels in the cancer immunity cycle. (A) ISV with immune adjuvant can cause local tissue damage, inducing immunogenic cell death and release of cancer cell antigens; and/or directly introduce PAMPs and/or DAMPs with TLR agonists or NPs into the tumor to (B) attract and activate dendritic cells and other antigen-presenting cells, (C) which mature and migrate from primary tumor to stimulate the priming and activation process of T cells in the tumor-draining lymph nodes. (D) Once primed and activated, T cells will then traffick to and (E) infiltrate solid tumors and metastases to (F) directly kill the tumors with granzymes and perforins. Multiple ISV strategies can be combined to achieve stronger anti-tumor immune response. ICD: immunogenic cell death. TAAs: tumor associated antigens. Immune adjuvants: injection of immune stimulating reagents. DAMPs: danger-associated molecular pattern molecules. PAMPs: pathogen associated molecular pattern molecules. HIFU: high intensity focused ultrasound. TLR: toll like receptor. NP: nanoparticles. Adapted from Chen and Mellman [Citation52].](/cms/asset/e4a5d61f-f02b-49ef-9150-301ced198a86/ihyt_a_1810333_f0002_c.jpg)
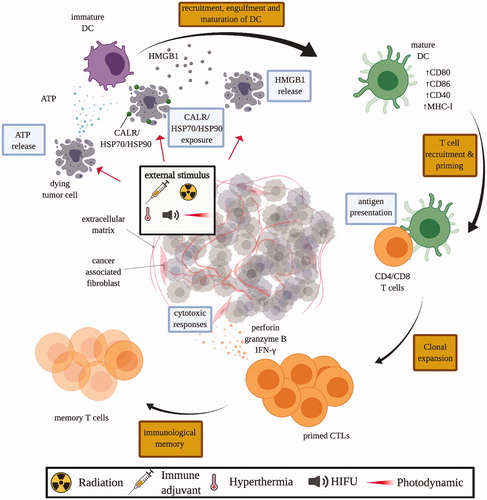
Figure 3. Mechanisms of immunogenic cell death (ICD). Tumor cells subjected to ICD-inducing treatment, such as chemotherapeutics, immune adjuvant injection, radiation therapy, HIFU, and photodynamic therapy, release damage-associated molecular pattern (DAMP) molecules like calreticulin (CALR), adenosine triphosphate (ATP), heat shock protein 70 and 90 (HSP70/90), and high mobility group box 1 (HMGB1). These DAMP molecules stimulate the recruitment and maturation of dendritic cells (DCs), which facilitates the engulfment of tumor antigens, and antigen presentation to T cells in the draining lymph nodes. Consequently, matured DCs trigger the priming of an adaptive immune response against tumor cells by T cells. Primed cytotoxic T lymphocytes (CTLs) expand and leave the lymph nodes that drain the treated tumors, circulate and identify other tumors by antigen expression, and kill untreated tumors with cytoxic effector molecules like perforin-1 and granzyme B.