Figures & data
Figure 1. Integrated stress response affects cell functioning Amino acid deficiency, heme deficiency, viral infection, and unfolded protein stress in the endoplasmic reticulum (ER stress) activate GCN2, HRI, PKR, and PERK. All these kinases phosphorylate eIF2α, the core member of ISR. Phosphorylated eIF2α (peIF2α) impedes the 5ʹcap-dependent mRNA translation ( in details) and results in global reduction of protein synthesis. However, few genes as ATF4 and CHOP have alternative translation machinery and are less influenced by eIF2 dysfunction. The preferentially translated ATF4 is the key effector in ISR. ATF4 couples with another ATF4 or its interacting partners to form homo- and heterodimers that bind to DNA targets and control the expression of genes that participated in amino acid synthesis, angiogenesis, ERAD, autophagy, inflammation, and apoptosis. Depends on the disease context and the duration of ISR, the downstream processes of ISR can aid in cell survival or bring cell death. CHOP is one key factor that can be promoted by ATF4. CHOP not only activates autophagy, inflammation, and apoptosis, but also induces the expression of GADD34, a phosphatase coupled with PP1 to dephosphorylate peIF2α. The dephosphorylation of peIF2α terminates ISR and resumes protein synthesis. At non-stressed cell, the CreP-PP1 complex phosphatase constantly operates for maintaining low level of peIF2α and protein homeostasis
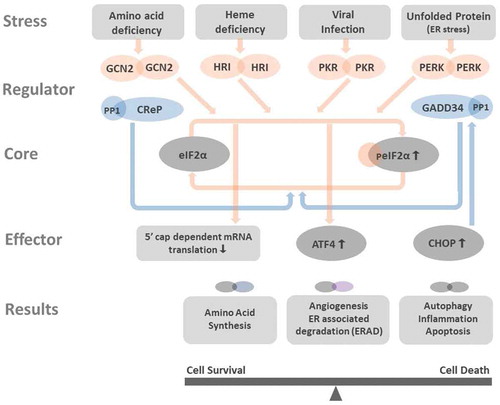
Figure 2. Illustration of the roles of eIF2 and peIF2 in the initiation of mRNA translation Before binding to met-tRNA, the GDP bound to the γ subunit of eIF2 must be switched to GTP. This GDP-GTP exchange process is mediated by eIF2B. The met-tRNA-GTP-eIF2 ternary complex then delivers the met-tRNA to 40S ribosome, a key step for the initiation of mRNA translation. At the stressed condition, once the ISR is activated and the GDP-eIF2 is phosphorylated in its α subunit, the structural change makes GDP-peIF2 no longer a suitable substrate of eIF2B but transforms to an inhibitor of eIF2B. The inhibition of eIF2B by GDP-peIF2 further reduces the pool of functional GTP-eIF2 to bind met-tRNA, and lowers the global protein synthesis via 5ʹcap-dependent mRNA translation. ISRIB rescues protein translation by stabilization and increasing eIF2B abundance that counteracts low level of GDP-peIF2α in treated cells
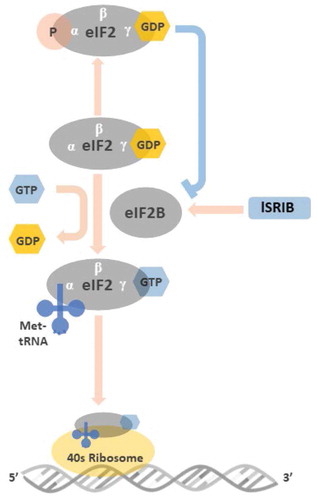
Figure 3. Targeting the druggable ISR pathways Modulation of ISR can be achieved by: (1) Targeting the eIF2α kinases: the GCN2 activators include Indirubin-3ʹ monoxime, Sp600125, Sky inhibitor; the GCN2 inhibitors consist of halofuginone, histidinol, asparaginase, arginine deiminase. The HRI activator BtdCPU and the inhibitor, amino-pyrazolindine. The PKR activators are BEPP and Poly (I:C). The PKR inhibitors are C16 and 2-aminopurine. The selective PERK activator is CCT020312 and the inhibitors are GSK2606414 and its more selective derivative GSK2656157. (2) Targeting the peIF2α phosphatase: Salubrinal and SAL003 inhibit both the inducible phosphatase GADD34 and the constitutive expressed CReP. In addition, Sephin 1 and Guanabenz inactivate GADD34, while Nelfinavir inactivates CReP. (3) Antagonist to the peIF2α: as shows, peIF2α inhibits the eIF2B. ISRIB is hypothesized to stabilize eIF2B and offsets the peIF2α effects while the peIF2α concentration is low. (4) Inhibition of ATF4 post-translational phosphorylation: RSK2 and PKA are two kinases that phosphorylate ATF4 and increase its activity. The RSK2 inhibitors, SL0101, BI-D1870, fmk and the PKA inhibitors, H-89, KT 5720, Rp-8-Br-cAMPS are potential compounds that inhibit ATF4 phosphorylation. (5) Targeting pathways downstream of ATF4: among the cellular functions regulated by ATF4, angiogenesis, autophagy and ERAD played important roles in ocular homeostasis and pathogenesis such as dry eye, diabetic retinopathy, age-related macular degeneration. Bortezomib, a proteasome inhibitor impedes ERAD has been demonstrated with neuro-protective and anti-inflammatory effects in vivo.
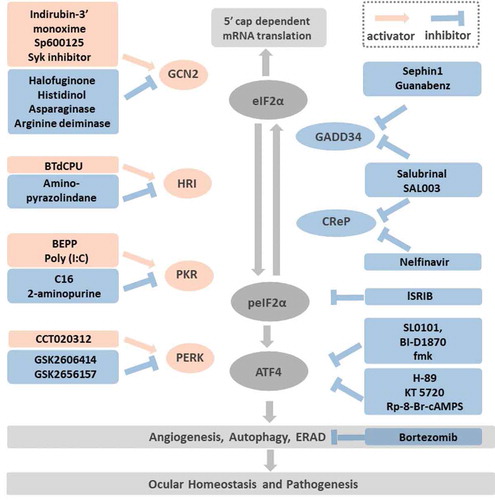
Table 1. Consequence of the genetic manipulation of ISR components in mice
