Figures & data
Table 1. PROs and PROMs identified in the literature review.
Table 2. PROs and PROMs classification proposed by nominal groups.
Figure 3. Core group of measurement instruments in order of priority, PROs considered relevant for assessment of severe asthma, and frequency of measurement.
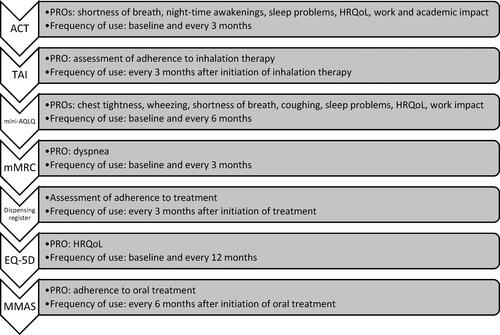
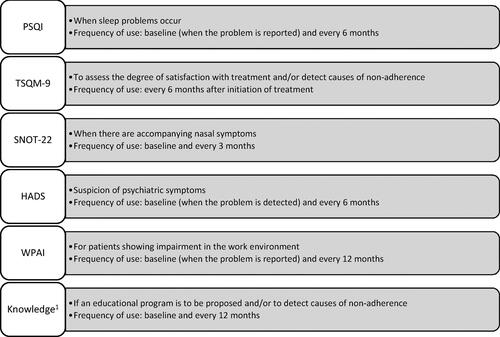
Figure 4. Complementary PROMs recommended for use in certain circumstances or interventions related to severe asthma and frequency of measurement.