Figures & data
Table I. Distribution of patient characteristics by risk strata and allocated treatment
Table II. Analysis of first events and cause specific mortality among all patients
Figure 2. Overall and event-free survival among all patients according to allocated treatment. Relative hazard (HR) and logrank p-values are indicated.
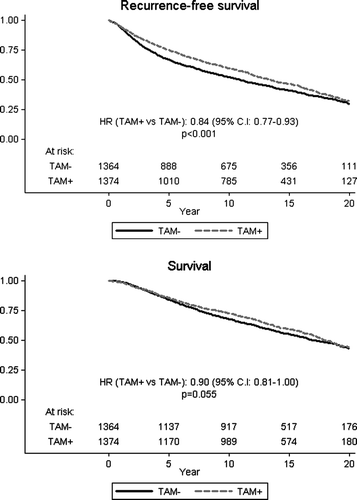
Table III. Analysis of events by period of follow-up
Table IV. Analyses of the interaction between ER-content and the effect of adjuvant tamoxifen
Table V. Effect of tamoxifen by ER and PgR-status according to assay technique
Table VI. Analysis of first events and cause specific mortality among ER positive patients
Figure 3. Overall and event-free survival among ER positive, high risk risk patients according to allocated treatment.
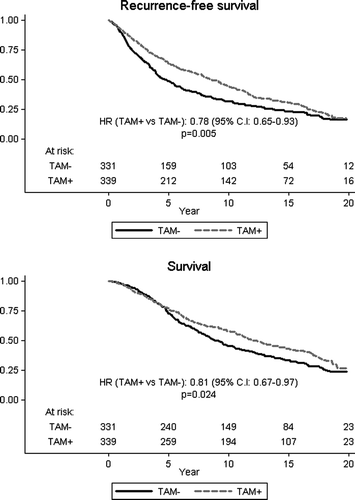
Figure 4. Overall and event-free survival among ER positive, low risk risk patients according to allocated treatment.
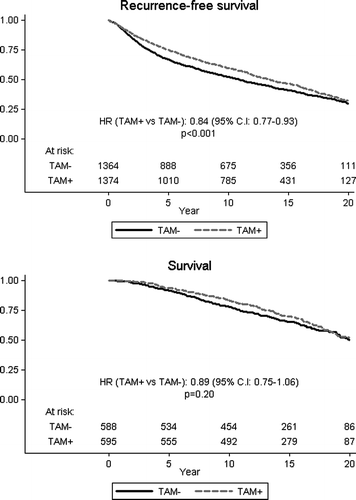
Table VII. Analysis of first events and cause specific mortality among patients randomly allocated to 2 versus 5 years of tamoxifen
Table VIII. Analysis of first events and cause specific mortality among patients randomly allocated to 2 or 5 years of tamoxifen, and concurrent patients allocated to no adjuvant endocrine therapy
Table IX. Number of new cancers (other than contralateral breast cancer) diagnosed as first events by allocated treatment
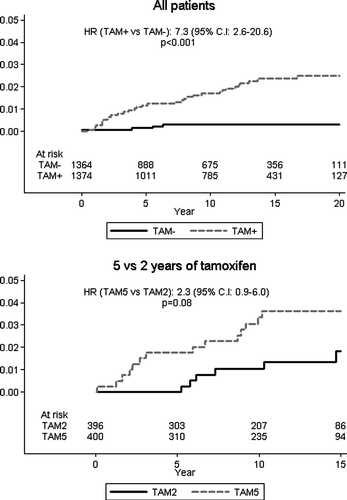
Figure 5. Cumulative incidence of endometrial cancer according to allocated treatment among all patients (left panel), and among those included in the randomized comparison of 2 versus 5 years of tamoxifen (right panel). Note that in the latter trial, the starting point for the curves is at 2 years after the initial entry into the trial. Number of patients at risk, relative hazards, and logrank p-values are indicated.