Figures & data
Table I. Characteristics of studies included in the meta-analysis.
Figure 1. Annotated forest plot for meta-analysis of the incidence of hand-foot skin reaction (HFSR) in cancer patients who received sorafenib. The summary incidences of all-grade (A) and high-grade (B) HFSR are calculated using a random-effects model. The incidences and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial.
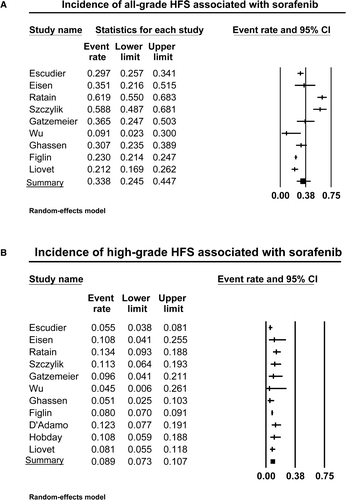
Figure 2. Annotated forest plot for meta-analysis of the incidence of hand-foot skin reaction (HFSR) associated with sorafenib in patients with renal cell cancer. The summary incidences of all-grade (A) and high-grade (B) HFSR associated with sorafenib for patients with RCC are calculated using a random-effects model. The incidences and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial.
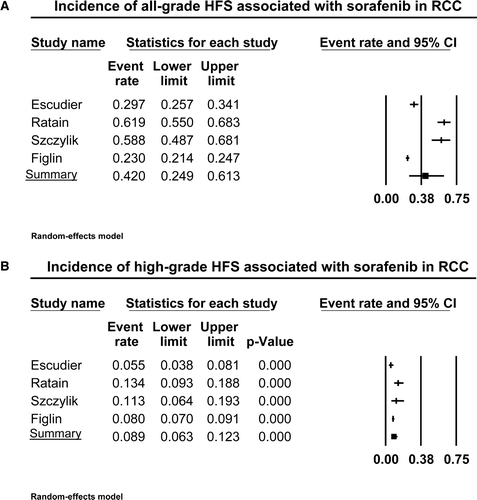
Figure 3. Annotated forest plot for meta-analysis of the incidence of hand-foot skin reaction (HFSR) associated with sorafenib in patients with non-renal cell cancer. The summary incidence of all-grade (A) and high-grade (B) HFSR is calculated using a random-effects model. The incidences and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial.
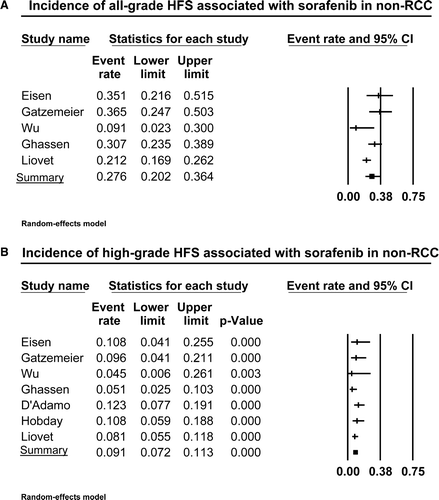
Figure 4. Relative risk (RR) of hand-foot skin reaction associated with sorafenib versus control in patients with metastatic renal cell carcinoma and hepatocellular carcinoma. The summary RR was calculated using a random-effects model. RR and 95% confidence intervals for each study and the final combined result are displayed numerically on the left and graphically as a forest plot on the right. Under study name, the first author's name was used to represent each trial.
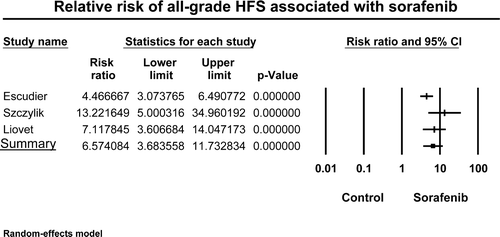
Table II. Association of hand-foot skin reaction with molecular targets.
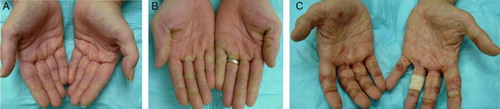
Figure 5. Example of grades I (A), II (B), III (C) hand-foot skin reaction (HFSR) in patients receiving sorafenib.