Figures & data
Table I. Patient characteristics.
Figure 1. Disease-free survival (DFS) comparison between the 77 and 82 programs (Panel A) and the 77 and 89 programs (Panel B).
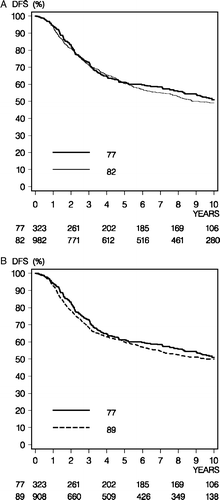
Table II. End-points.
Figure 2. Overall survival (OS) comparison between the 77 and 82 programs (Panel A) and between the 77 and 89 programs (Panel B).
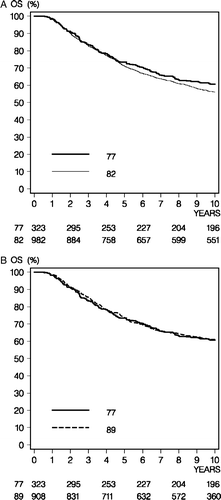
Figure 3. Forest plots illustrating proportional hazard models for DFS comparison between the 77 and 82 programs (Panel A) and between the 77 and 89 programs (Panel B). Hazard ratios refer to adjusted per protocol estimates obtained in the multivariate analysis.
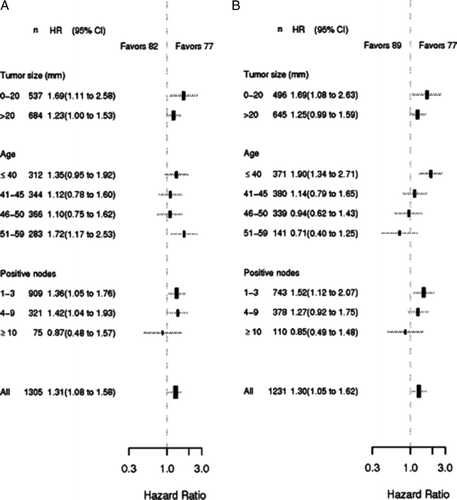
Figure 4. Forest plots illustrating proportional hazard models for OS comparison between the 77 and 82 programs (Panel A) and between the 77 and 89 programs (Panel B). Hazard ratios refer to adjusted per protocol estimates obtained in the multivariate analysis.
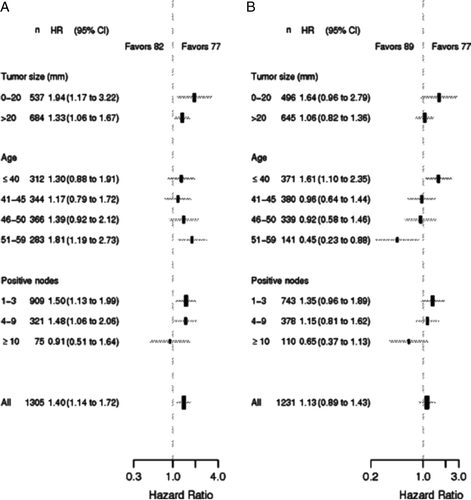
Figure 5. Delivered dose-intensity of cyclophosphamide (Panel A), methotrexate (Panel B) and fluorouracil (Panel C) (weekly dose in mg/m2) in the 77 (•) 82 (▪) and 89 (▴) programs.
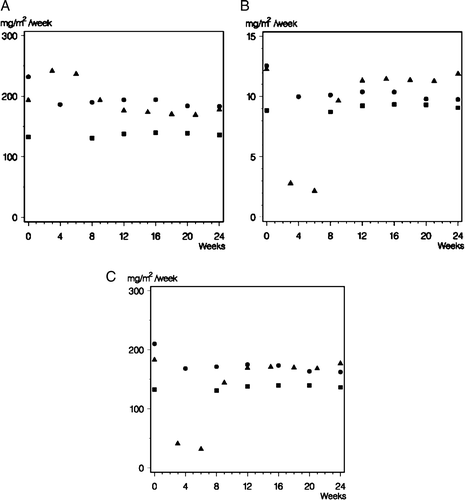
Table III. Treatment-related adverse effects (Modified WHO criteria).
Table IV. Planned dose-intensity.
