Figures & data
Table I. Patient characteristics.
Table II. Patient pre treatment prior to enter in the study.
Table III. Number of adverse events with > 10 occurrences and their CTC grade, whether the AEs were related to study treatment or not.
Table IV. Mean (SD) PK parameters for CP-4055, ara-C and ara-U for all dose levels on Day 1.
Figure 1. Concentrations of CP-4055 (♦), ara-C (▪) and ara-U (•) after a 30 min (—) and 2 hr (---) infusion of CP-4055 at dose level 240 mg/m2.
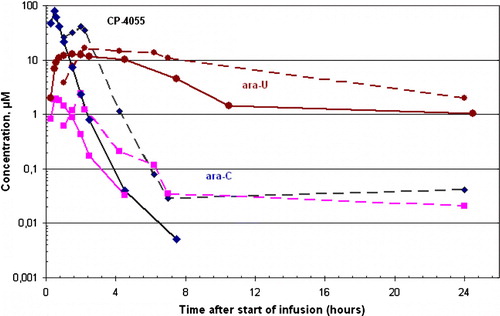
Table V. Occurrence and duration of partial response and stable disease by tumour type and CP-4055 dose.