Figures & data
Figure 1. Flowsheet over patients included in the present paper. The 1st sub-analysis was done to evaluate a possible role of ApoDCN co-expression with ERα in the elderly patients ≥ 70 years. The 2nd sub-analysis evaluated the potential influence of ApoDCN status on the predictive effect of tamoxifen in the ERα positive postmenopausal patients with node positive disease included in the NBCG-1 trial [13]. DCIS, ductal carcinoma in situ; y, years; ERα, estrogen receptor alfa; ApoDCN, Combined cytoplasmic and nuclear ApoD staining; pN+, node positive; NBCG-1, Norwegian Breast Cancer Group adjuvant study # 1 [13].
![Figure 1. Flowsheet over patients included in the present paper. The 1st sub-analysis was done to evaluate a possible role of ApoDCN co-expression with ERα in the elderly patients ≥ 70 years. The 2nd sub-analysis evaluated the potential influence of ApoDCN status on the predictive effect of tamoxifen in the ERα positive postmenopausal patients with node positive disease included in the NBCG-1 trial [13]. DCIS, ductal carcinoma in situ; y, years; ERα, estrogen receptor alfa; ApoDCN, Combined cytoplasmic and nuclear ApoD staining; pN+, node positive; NBCG-1, Norwegian Breast Cancer Group adjuvant study # 1 [13].](/cms/asset/0617a0a1-7487-47c5-8df5-2f945b485dbf/ionc_a_362229_f0001_b.gif)
Table I. Comparison of features between subgroups of ERα /ApoDCN status (n = 290).
Figure 2. A. Relapse free survival (RFS; all locations) of the elderly patients ≥ 70 years. Grouping is based on ApoDCN and ERα expression in the cytoplasm. A significant difference in RFS (p = 0.004) was found between group 1 (ERα + /ApoDCN−) and group 2 (ERα + /ApoDCN+). Of note is the observation of no significant difference (p=0.39) in RFS between group 2 (ERα + /ApoDCN+) and group 3 (ERα − /ApoDCN+). We excluded the ERα − /ApoDCN− tumors from the analysis in this age group, due to a low number of patients (n = 4).B. Breast cancer specific survival (BCSS; dead of breast cancer) in the elderly patients > 70 years. Stratification is based on ERα/ApoDCN category of the primary tumor. A statistically significant survival difference (p = 0.004) between group 1 (ERα + /ApoDCN−) and group 2 (ERα + /ApoDCN+) was observed, and also between group 2 and group 3 (ERα − /ApoDCN+) (p = 0.03). We excluded the ERα − /ApoDCN− tumors from the analysis in this age group, due to a low number of patients (n = 4).
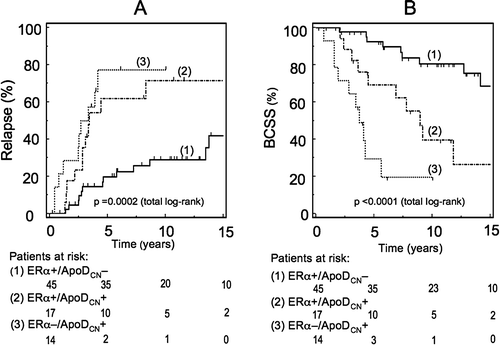
Table II. Univariate survival analysis of patients ≥70 years of age (n = 76)
Table III. Multivariate analysis of patients ≥70 years of age (n = 72)
Figure 3. A. Breast cancer specific survival (BCSS) in the ApoDCN− tumors of postmenopausal patients enrolled in a prospective randomized adjuvant study (Adjuvant study NBCG − 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].B. Breast cancer specific survival (BCSS) in the ApoDCN+ tumors of postmenopausal patients enrolled in the prospective randomized adjuvant study (Adjuvant study NBCG – 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].
![Figure 3. A. Breast cancer specific survival (BCSS) in the ApoDCN− tumors of postmenopausal patients enrolled in a prospective randomized adjuvant study (Adjuvant study NBCG − 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].B. Breast cancer specific survival (BCSS) in the ApoDCN+ tumors of postmenopausal patients enrolled in the prospective randomized adjuvant study (Adjuvant study NBCG – 1) on 20 mg tamoxifen (TAM) for 2 years vs. placebo (i.e. Control = Ctr) [13].](/cms/asset/33cd51d7-8449-497f-9bc1-c3846d828699/ionc_a_362229_f0003_b.gif)
