Figures & data
Figure 1. Cell lysates were separated by electrophoresis: lane 1–6 in a 7% SDS-PAGE gel according to Laemmli, and lane 7–10 in a 4–12% NuPAGE, Bis-Tris gel with MOPS buffer. Lanes 1–5 and 7–9 were probed with anti-EpoR C-20 and lanes 6 and 10 with H-194. UT-7/Epo cells were grown with 2×103 IU/L rHuEpo β (lanes 1 and 7) or without rHuEpo β for 24 h (lanes 2 and 8). Alternatively, they were stimulated for 10 min (lane 3) or 60 min (lane 4) with 10×103 IU/L rHuEpo β. LU-HNSCC-7 cells were grown with 10% FCS (lanes 5, 6, 9 and 10). Band b, c and d have earlier been identified in UT-7/Epo as different forms of EpoR while band a and e were unrelated to EpoR Citation[20]. Band f and g were present in LU-HNSCC-7.
![Figure 1. Cell lysates were separated by electrophoresis: lane 1–6 in a 7% SDS-PAGE gel according to Laemmli, and lane 7–10 in a 4–12% NuPAGE, Bis-Tris gel with MOPS buffer. Lanes 1–5 and 7–9 were probed with anti-EpoR C-20 and lanes 6 and 10 with H-194. UT-7/Epo cells were grown with 2×103 IU/L rHuEpo β (lanes 1 and 7) or without rHuEpo β for 24 h (lanes 2 and 8). Alternatively, they were stimulated for 10 min (lane 3) or 60 min (lane 4) with 10×103 IU/L rHuEpo β. LU-HNSCC-7 cells were grown with 10% FCS (lanes 5, 6, 9 and 10). Band b, c and d have earlier been identified in UT-7/Epo as different forms of EpoR while band a and e were unrelated to EpoR Citation[20]. Band f and g were present in LU-HNSCC-7.](/cms/asset/604be537-cfb2-4688-96f0-b8ce70523f05/ionc_a_391527_f0001_b.gif)
Figure 2. Separation of anti-EpoR reactive proteins by two-dimensional electrophoresis. First dimension: SDS-PAGE according to Laemmli, second dimension: 4–12% NuPAGE, Bis-Tris with MOPS buffer. A. Epo starved UT-7/Epo cells probed with C-20. B. Serum starved LU-HNSCC-7 cells probed with C-20. C. LU-HNSCC-7 cells probed first with C-20 and then, after stripping, with H-194. The images were overlaid electronically and matched using the membrane outlines. The background in the H-194 image was subtracted so that only the immunoreactive spot was visible (arrow g). Spot a and c-g correspond to band a and c-g in , i.e. spot c and d represent different forms of EpoR. Spot f and g, present mainly in LU-HNSCC-7, were separate from the EpoR spots.
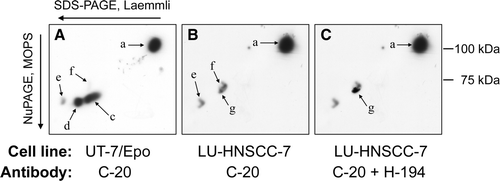
Figure 3. RT-PCR of EpoR mRNA. PCR products from all (3×3) replicates of each combination of cell line and growth condition were pooled and separated by polyacrylamide gel electrophoresis. UT-7/Epo cells grown without Epo for 24 h (lane 1), or with 2×103 IU/L rHuEpo (lane 2), and LU-HNSCC-7 cells (lane 3). In lane 4, 10-fold higher amount of the LU-HNSCC-7 product was loaded. GAPDH mRNA was probed for relative quantification.
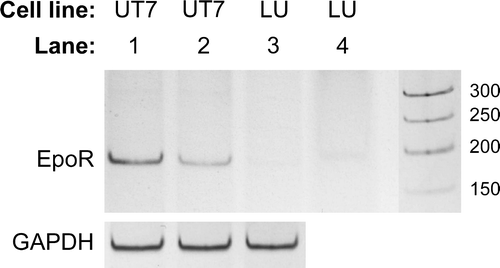
Figure 4. Cell lysates from UT-7/Epo and LU-HNSCC-7 cells stimulated with 10×103 IU/L rHuEpo β for the indicated times were separated by electrophoresis, transferred to PVDF membrane and probed consecutively with anti-phosho-JAK2, anti-phospho-STAT5 and anti-actin (A) or anti-phospho-Akt, anti-phospho-p44/p42 MAPK and anti pan-actin (B).
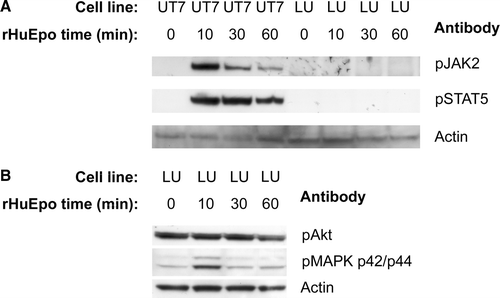
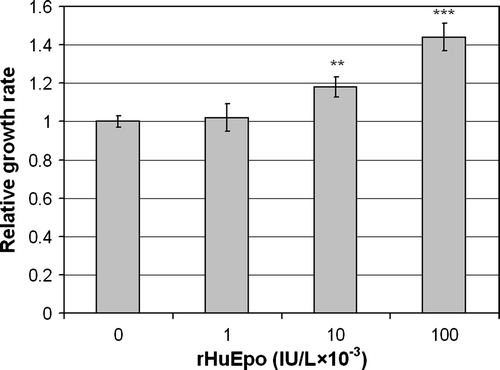
Figure 5. Effect of rHuEpo β on serum-deprived LU-HNSCC-7 cells. Cells were seeded in 60 mm dishes. Twenty-four hours later, the cells received new medium without serum but with the indicated amounts of rHuEpo β and were incubated for seven days before cell counting. The results were calculated as the average of four independent experiments with triplicate measurements. Error bars indicate standard error of the mean (SEM) (**p < 0.01 and ***p < 0.001).