Figures & data
Table 1. Patient characteristics of PDAC patients operated in 2000–2015 according to preoperative treatment.
Figure 1. A. DSS in operated PDAC patients (2010–2015) according to preoperative treatment. Median survival for neoadjuvant therapy was 35 (95% CI 25–44) months and for upfront surgery 26 (95% CI 20–31) months, p = .008. B. DFS in operated PDAC patients (2010–2015) according to preoperative treatment. Median survival for neoadjuvant therapy was 25 (95% CI 13–36) months and for upfront surgery 13 (95% CI 6–21) months, p = .001.
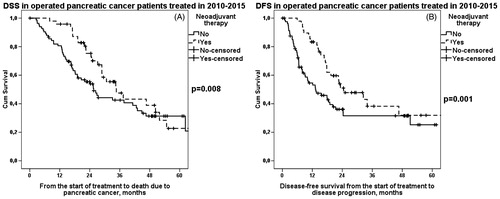
Table 2. DSS and DFS in operated PDAC patients according to preoperative treatment.
Figure 2. A. DSS in patients with grade 3 tumor according to preoperative treatment. Patients: n(NEO) = 14, n(US) = 26. Median survival for neoadjuvant therapy 30 (95% CI 17–42) months and for upfront surgery 11 (95% CI 8–15) months, p = .004. NEO: neoadjuvant therapy; US: upfront surgery. B. DFS in patients with grade 3 tumor according to preoperative treatment. Patients: n(NEO) = 14, n(US) = 26. Median survival for neoadjuvant therapy was 21 (95% CI 11–31) months and for upfront surgery 7 (95% CI 5–8) months, p = .001. NEO: neoadjuvant therapy; US: upfront surgery.
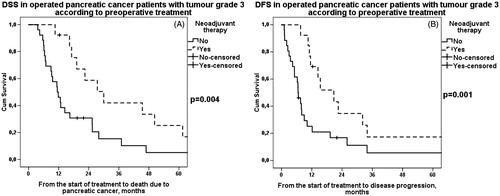
Figure 3. A. DSS in operated stage IIB–III pancreatic cancer patients according to preoperative treatment. Patients: n(NEO) = 35, n(US) = 107. Median survival for neoadjuvant therapy was 34 (95% CI 29–40) months and for upfront surgery 20 (95% CI 14–26) months, p = .006. NEO: neoadjuvant therapy; US: upfront surgery. B. DFS in operated stage IIB–III pancreatic cancer patients according to preoperative treatment. Patients: n(NEO) = 35, n(US) = 107. Median survival for neoadjuvant therapy was 21 (95% CI 12–29) months and for upfront surgery 10 (95% CI 7–13) months, p = .001. NEO: neoadjuvant therapy; US: upfront surgery.
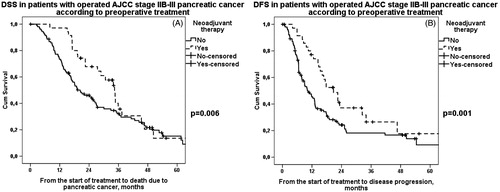
Table 3. DSS and DFS survival in operated PDAC patients according to different prognostic parameters and preoperative treatment.
