Figures & data
Table I. Mutations in HSPB1 and HSPB8 and the associated phenotypes.
Figure 1 Structure‐based sequence alignments of HSPB1 and HSPB8 orthologues and homologues HSPB4 and HSPB5. The N‐terminal WD/EPF and SRLDQF/AFG domain, the α‐crystallin domain and the C‐terminal IXI/V domain are indicated. The peripheral neuropathy‐associated mutations are boxed in grey. The cytochrome C binding motifs are indicated by a dotted line.
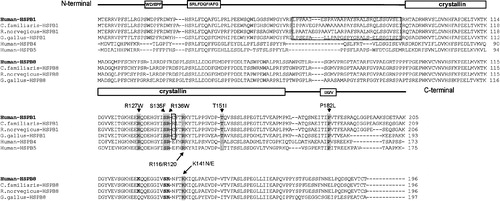
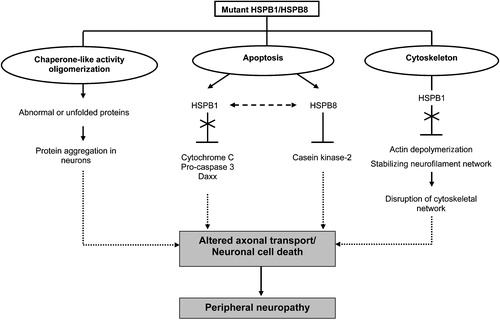
Figure 2 Suggested pleiotropic actions of mutant HSPB1 and HSPB8. Mutations in HSPB1 and HSPB8 can interfere with the chaperone‐like activity, causing insoluble cellular aggregates and subsequent induction of neuronal cell death. HSPB1 and HSPB8 regulate different apoptotic pathways. Mutant HSPB1 can no longer bind cytochrome C, pro‐caspase 3 and Daxx and is thus unable to prevent apoptotic cell death. Mutations in HSPB8 may result in enhanced apoptosis by inhibiting the pro‐survival activity of casein kinase‐2 or by negatively influencing the anti‐apoptotic activity of its interacting partner, HSPB1. Furthermore, mutant HSPB1 is able to disrupt cytoskeletal functions and neurofilament assembly, resulting in neuronal cell death or altered axonal transport. Ultimately, these pathological mechanisms will cause peripheral neuropathy in humans.