Figures & data
Figure 1 Cellular interactions are important in allergic reactions. B cells develop from stem cells in the bone marrow. Naïve B cells that exit the bone marrow will travel to the lymph nodes for activation. Allergens are taken up by antigen‐presenting cells (e.g. dendritic cells) and presented to T cells via MHC class II, which become activated and proliferate into Th2 cells. When B cells encounter their specific antigen, it is presented on the surface of the B cell via MHC class II. The subsequent stimulation of specific Th2 cells leads to the production of IL‐4 and the upregulation of expression of CD40L by T cells. CD40 stimulation of allergen‐specific B cells upregulates the expression of the costimulatory molecules CD80 and CD86, which allows for more efficient T cell expression of CD40L and enhanced stimulation of B cells through the induction of IL‐4. CD40‐mediated stimulation of B cells synergizes with IL‐4 receptor signals to enhance rearrangement of the IgE genomic locus and production of IgE antibodies. Allergen‐specific IgE binds to the high‐affinity receptor for IgE (FcϵRI) on mast cells. Allergen exposure induces cross‐linking of receptor‐bound IgE with subsequent mast cell degranulation and the release of proinflammatory molecules such as histamine. Notes: BCR: B‐cell receptor; FcϵRI: High affinity IgE receptor; MHC: Major histocompatibility complex; TCR: T‐cell receptor.
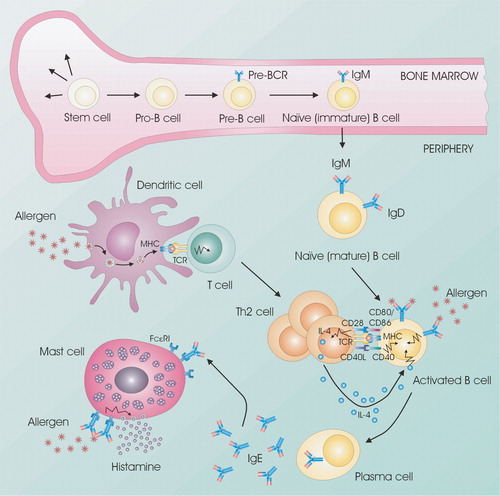
Figure 2 The combined results of various studies on age‐related reference values for serum IgE. Only studies using nonatopic populations have been included, but smoking habits and gender differences have not been taken into account. A weighted geometric mean (gm) for the individual gm and gm +2× SD have been calculated (SD based on A–E), and are shown with thick lines as ‘combined’. If other age intervals were used by the authors the total number of subjects in a group were evenly distributed in the appropriate age groups. In the lower panel is shown the number of patients in each age group. A total of 4104 subjects were used for the calculations. A: Citation153; B: Citation4; C: Citation154; D: Citation155; E: Citation156; F: Citation157; G: Citation158; H: Citation159.
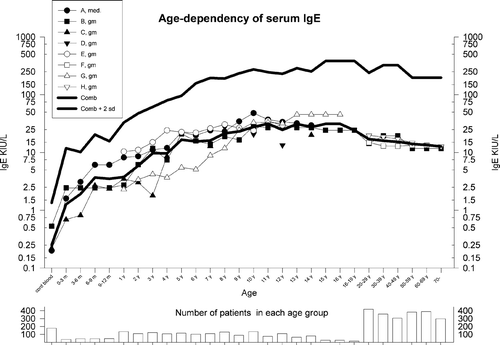
Figure 3 Breakdown of evidence for the hygiene hypothesis. It is suggested that evidence is divided into clinical/epidemiological versus immunological. Furthermore, studies of allergy versus autoimmunity can be studied (and results explained) independently of hygienic influences (‘Th1‐Th2 hypothesis’). Studies involving hygienic factors should make clear whether they are based on a Th1‐Th2 dichotomy (‘hygiene hypothesis’) or the concept of T cell regulation and tolerance induction (‘the revised hygiene hypothesis’) by means of regulatory T cells (Tr1/Th3/Treg).
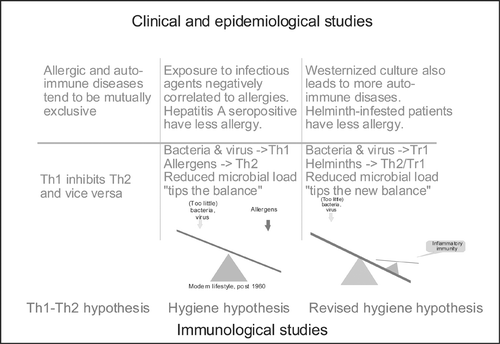
Figure 4 Tentative model of the differentiation of Thelper(CD4+) subsets. The Thelper phenotypes Th17 (producing IL‐17), Th1 (IFN‐γ), Th2 (IL‐4, IL‐5, and IL‐13), and various regulatory Th cells (IL‐10 and/or TGF‐β) are induced by dendritic cells secreting (IL‐6+TGF‐β), IL‐12, IL‐4, and (TGF‐β and/or IL‐10). Recent results suggest that Th1, Th2, and Tr1 can be subdifferentiated into IL‐10 or TNF‐α producers via the OX40‐OX40 ligand system. It is not known whether Th17 cells can be subdifferentiated this way. Notes: APC: Antigen presenting cell; DC: Dendritic cell; Dex+Vit D3: Dexamethasone plus vitamin D3; Foxp3: Forkhead box p3; GATA‐3: GATA binding protein 3; ROR‐γT: RAR‐related orphan receptor γ; STAT: Signal Transducers and Activators of Transcription proteins; T‐bet: T box transcription factor; TLR: Toll like receptor.
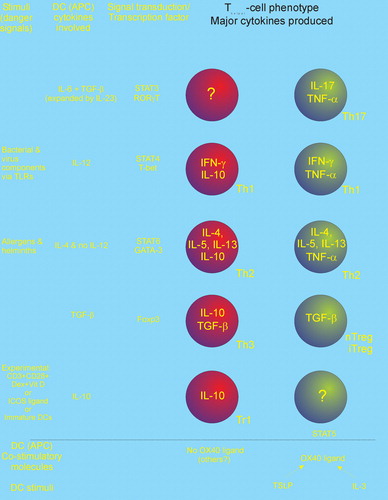
Table I. Pairs of costimulatory molecules on antigen‐presenting cells and T cells.
Figure 5 Rearrangement at the immunoglobulin heavy chain locus. The human immunoglobulin heavy chain locus contains clusters of heavy chain variable(V), diversity (D), and joining (J) cassettes that are rearranged during B cell development. This process results in the assembly of a complete rearranged VDJ region that encodes an antigen‐binding domain capable of producing intact μ and δ heavy chains. STAT‐6 and NF‐κB are activated upon stimulation with IL‐4 and aCD40, initiating the production of μ and ϵ GLTs resulting in CSR. CSR is a process that exchanges the constant region of the heavy chain (CH) with a set of downstream constant‐region genes (here CSR to IgE is shown). For IgE isotype switching, this process involves the excision of a large piece of genomic DNA spanning from Sμ sequences to the Sϵ sequence. Ligation of the VDJ sequences to the constant (C) ϵ locus gives rise to an intact ϵ heavy chain gene and the production of intact IgE antibodies.
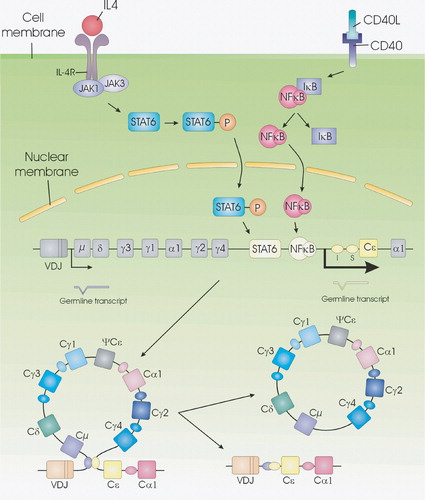
Figure 6 A model for the role of GLT in CSR. Upon B cell activation GLT is induced. This GLT interferes with the switch region forming a R‐loop to create a stable single‐strand DNA (ssDNA) substrate for AID. AID then converts dC to dU residues, creating targets for endonuclease resulting in DNA breakage necessary for CSR.
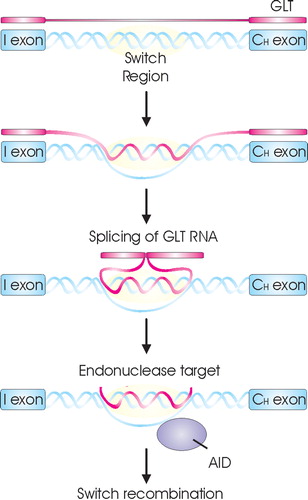
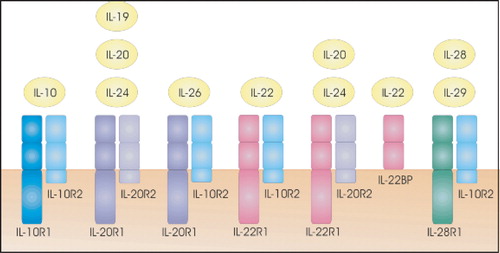
Figure 7 Receptors for IL‐10 and the IL‐10‐related cytokines. Cytokine receptor family class II members are receptors for IL‐10‐related cytokines.