Figures & data
Figure 1. Hydroxylation of proline residues by C-P4Hs is essential for the thermal stability of collagen triple helices. Non-hydroxylated collagen polypeptide chains cannot form functional molecules in vivo.
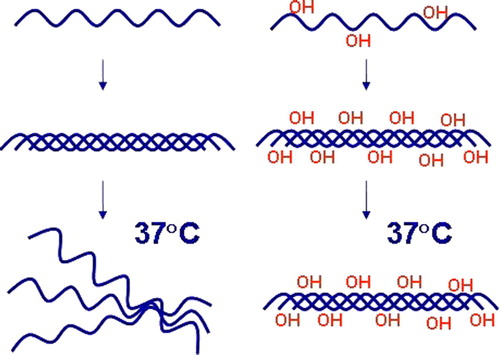
Figure 2. Regulation of the stability of the HIF-1α by oxygen-dependent prolyl 4-hydroxylation. Under normoxic conditions, HIF-1α is hydroxylated by HIF-P4Hs. Hydroxylation is required for binding of the VHL E3 ubiquitin ligase complex and for subsequent proteasomal degradation. Hypoxia inhibits the HIF-P4Hs, HIF-1α escapes degradation and forms a stable dimer with HIF-β. The dimer is translocated into the nucleus and binds to the HIF-responsive elements in a number of hypoxia-inducible genes.
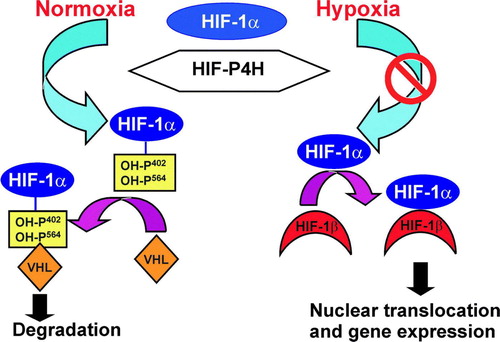
Figure 3. Schematic representation of the human C-P4H α(I), α(II) and α(III) subunits, P4H-TM and HIF-P4Hs 1–3. The lengths of the polypeptides are indicated on the right, and the catalytically critical residues are shown above the polypeptides. The peptide-substrate- binding domain in the C-P4H α subunits and the TM domain of P4H-TM are also indicated.
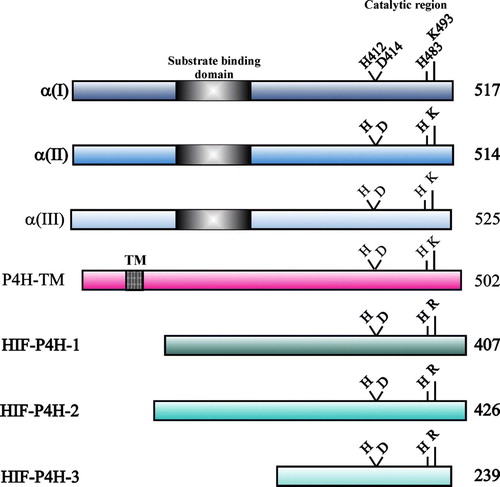
Figure 4. Reactions catalyzed by P4Hs. 2-Oxoglutarate is stoichiometrically decarboxylated during the hydroxylation reaction, which does not require ascorbate (A). The enzymes also catalyze the uncoupled decarboxylation of 2-oxoglutarate without subsequent hydroxylation of the peptide substrate. Ascorbate is stoichiometrically consumed in the uncoupled reaction, which may occur in either the presence (B) or absence (C) of the peptide substrate.]
![Figure 4. Reactions catalyzed by P4Hs. 2-Oxoglutarate is stoichiometrically decarboxylated during the hydroxylation reaction, which does not require ascorbate (A). The enzymes also catalyze the uncoupled decarboxylation of 2-oxoglutarate without subsequent hydroxylation of the peptide substrate. Ascorbate is stoichiometrically consumed in the uncoupled reaction, which may occur in either the presence (B) or absence (C) of the peptide substrate.]](/cms/asset/c6874f54-68b4-494f-8547-4c2155c0e31e/iann_a_298825_f0004_b.gif)
Figure 5. Schematic representation of the catalytic site of the α(I) subunit of human C-P4H-I. Fe2 + is coordinated with the enzyme by His412, Asp414, and His483. Subsite I of the 2-oxoglutarate binding site consists of Lys493, which ionically binds the C5 carboxyl group of 2-oxoglutarate, while subsite II consists of two cis-positioned equatorial coordination sites of the enzyme-bound Fe2 + and is chelated by the C1 carboxyl and C2 oxo functions of the 2-oxoglutarate. Molecular oxygen is bound end-on in an axial position, producing a dioxygen unit.
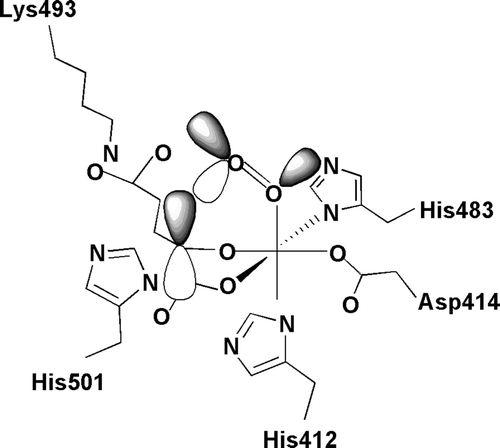
Table I. Km values of recombinant human C-P4Hs I-III and HIF-P4Hs 1-3 for Fe2 + , 2-oxoglutarate, O2 and ascorbatea.
Table II. Inhibition of purified recombinant C-P4H-I and HIF-P4Hs 1-3 by certain metals and 2-oxoglutarate analoguesa.
