Figures & data
Figure 1. Deep skin biopsy of the left lower leg of a 79 year-old male patient with non-uremic calciphylaxis. The von Kossa stain demonstrates vascular perieccrine calcification (arrow), calcification of a subcutaneous artery and adjacent capillaries (asterisk) as well as diffuse calcification of fibers in the deep dermis.
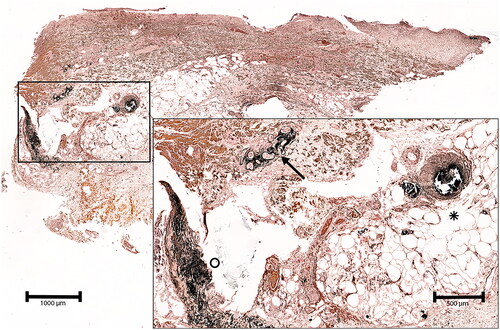
Table 1. Epidemiological characteristics of all calciphylaxis cases.
Figure 2. Overview of comorbidities of CUA vs. NUC patients at the time of diagnosis PAD = peripheral artery disease.
*Significant (p < 0.05) difference between the NUC and CUA cohorts.
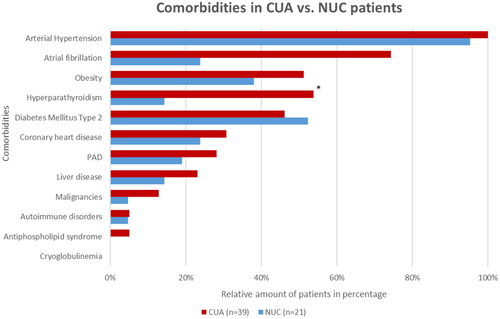
Figure 3. Overview of medication history of CUA vs. NUC patients at the time of diagnosis.
*Significant (p < 0.05) difference between the NUC and CUA cohorts.
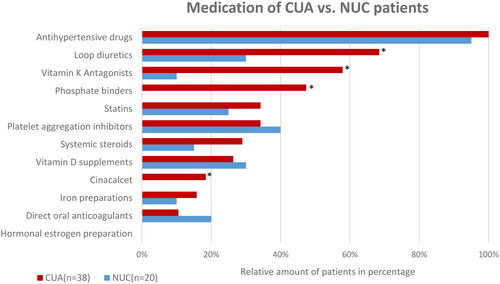
Table 2. Overview of laboratory abnormalities in CUA vs. NUC patients. Normal ranges for selected parameters: serum calcium 2.2 –2.65 mmol/l, serum phosphate 0.81 – 1.45 mmol/l, calcium-phosphorus-product < 4.40 mmol2/l2 and serum albumin 35—55 g/l.
Table 3. Systemic treatment in patients with NUC and CUA.
Data availability statement
The abstract contains 237 words, and the major text of the article contains 3261 words, three tables, three figures, and 61 references.
