Figures & data
Figure 1. Possible immune responses mechanism in stunted children with STH infection. IGF-1 is the key mediator of the action of the GH axis on linear growth. In stunted children, there is a disturbance in IGF-1. When IGF-1 is disrupted, there is a decrease in IL-4 levels, which can lead to chronic infection. Epithelial and endothelial barriers are damaged by helminth parasite infection, which induces a wound-repair and anti-parasite immune response that is driven by the type 2 cytokines interleukin-4 (IL-4), IL-5, and IL-13. Dendritic cells (DCs), basophils, natural killer T (NKT) cells, eosinophils, and group 2 innate lymphoid cells (ILC2s) function to produce the key TH2 cells. B cells also participate in secondary type 2 responses. Type 2 cytokines, in turn, target epithelial cells, goblet cells, smooth muscle cells, and macrophages, which together coordinate parasite expulsion by increasing fluid and mucus production, encapsulation and barrier formation, epithelial cell turnover, smooth muscle contraction, and the production of anti-parasite effector molecules such as resistin-like molecule-β (RELMβ). In addition to activating several anti-parasite effector mechanisms, the type 2 immune response facilitates wound repair, which is important following infection by these large multicellular tissue-invasive organisms. M2 macrophages are intimately involved in this process as they produce matrix metalloproteinases (MMPs), arginase 1 (ARG1), insulin-like growth factor 1 (IGF1), vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGFβ), which together promote myofibroblast activation, angiogenesis, epithelial cell turnover, and extracellular matrix (ECM) deposition. The helminth-induced type 2 immune response also promotes effective wound healing by suppressing the pro-inflammatory axis that is mediated by M1 macrophages, which could further exacerbate tissue injury if not quickly controlled. M2 macrophages producing TH2 cells have been shown to have important roles in the suppression of this pro-inflammatory axis and can also control potentially harmful type 2 immune responses. Macrophages and mature DCs can induce both adaptive T-lymphocyte-mediated and B-lymphocyte-mediated immunity by internalizing and processing pathogens. Macrophages and mature DCs also express the vitamin-D-activating enzyme CP27B and are thus able to synthesize 1.25(OH)2D from precursor 25OHD. The 1.25(OH)2D synthesized in this way can act in a paracrine fashion on activated B lymphocytes and activated T lymphocytes, which express abundant VDR. The dotted red line shows conditions when vitamin D deficiency occurs, and the purple line indicates when vitamin D is sufficient.
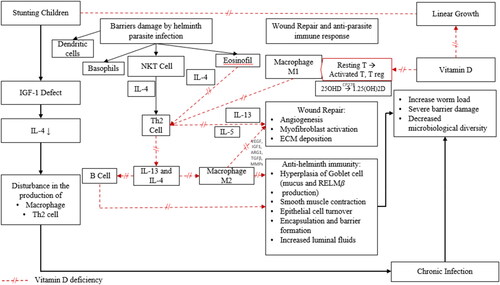
Table 1. Characteristics of stunted children aged 24–59 months with or without soil-transmitted helminth (STH) infection.
Table 2. Serum 25(OH)D levels of stunted children aged 24–59 months with soil-transmitted helminth (STH) infection.
Figure 3. Differences in interleukin-5 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
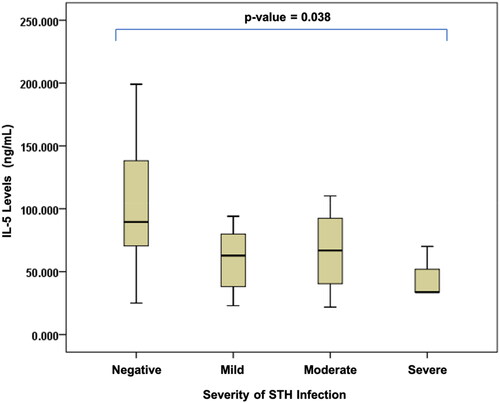
Figure 4. Differences in interleukin-13 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
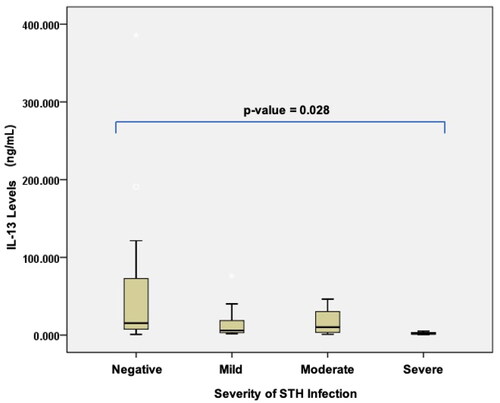
Table 3. Comparison of 25(OH)D, interleukin-4, interleukin-5, and interleukin-13 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
Table 4. Correlations of interleukin-4, interleukin-5, and interleukin-13 with vitamin D during soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.