Figures & data
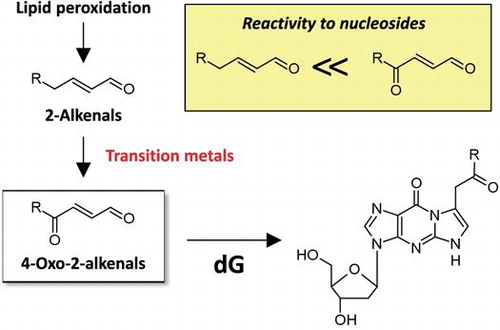
Fig. 1. LC-MS/MS analysis of 7-(2-oxo-hexyl)-εdG (OOE-dG) and 2-alkenal-derived 7-(2-oxo-alkyl)-εdG adducts.
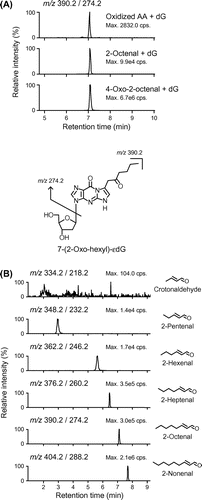
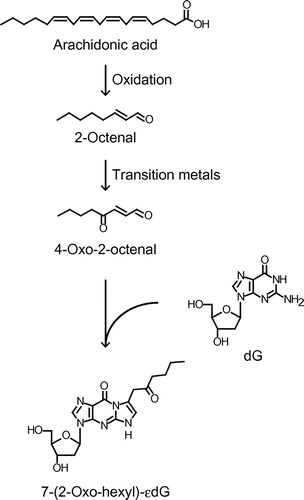
Fig. 2. Effects of transition metals on the formation of OOE-dG.
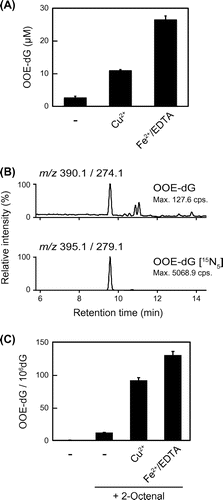
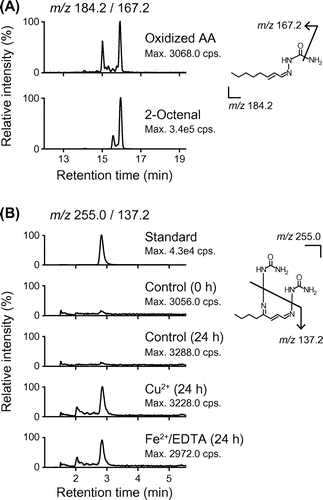
Fig. 3. LC-MS/MS analysis of semicarbazone derivatives of 2-octenal and OOE.