Figures & data
Figure 1. An adalimumab dose reduction and withdrawal protocol by extending the dosing interval. (Adalimumab 20 mg for the patient less than 30 Kg and 40 mg for those over 30 Kg).
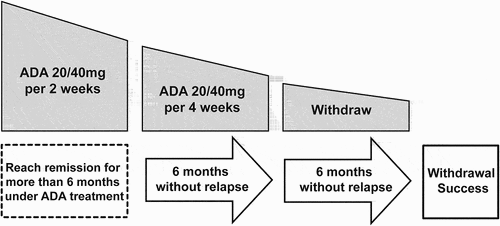
Table 1. Demographic characteristics of children with stable non-infectious uveitis.
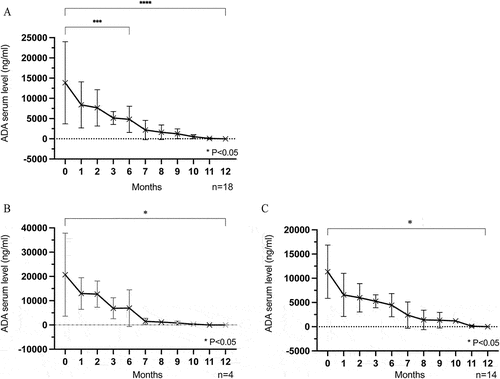
Figure 2. Kaplan-Meier survival curves showing relapse-free survival of all stable NIU patients after ADA dose reduction and withdrawal during the follow-up time. n = patients.
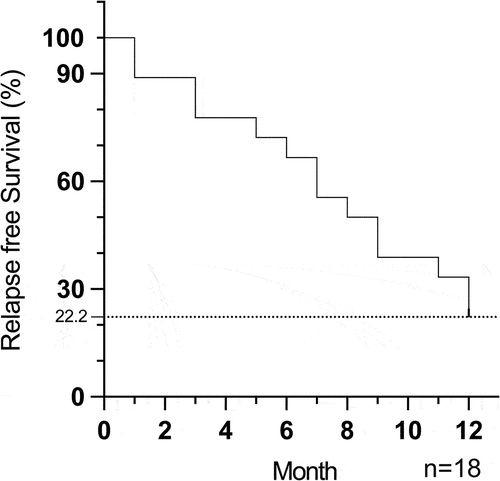
Table 2. Clinical and treatment features of non-infectious uveitis children before and after adalimumab-dose reduction and withdrawal.
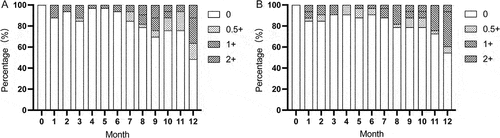
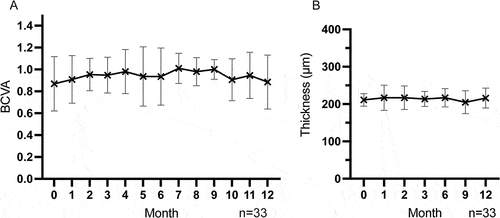
Figure 3. Change of BCVA (A) and CMT (B) after ADA dose reduction and withdrawal during the follow-up time. BCVA: best corrected visual acuity, CMT = central macular thickness. n = eyes.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.