Figures & data
Table 1. Characteristics of the Study Populationa.
Figure 1. Distribution of patients according to drug type and reason for discontinuation. IL-23 indicates interleukin-23. aTreatment was stopped as rheumatologists suspected psoriatic arthritis and treatment with ixekizumab was initiated instead. bThe patient stopped treatment due to an injection site reaction, headaches, and abdominal pain. cThe patient stopped treatment despite showing a good response and was switched to 150 mg risankizumab as the patient was receiving tildrakizumab in double dosage due to incomplete clearance at 100 mg. dOne patient had an injection site reaction and another experienced exanthema on the trunk and extremities following the 2nd injection and later also developed a petechial rash on arms and thorax. eOne stopped due to a desire for pregnancy and one stopped due to reevaluation of the psoriasis diagnosis.
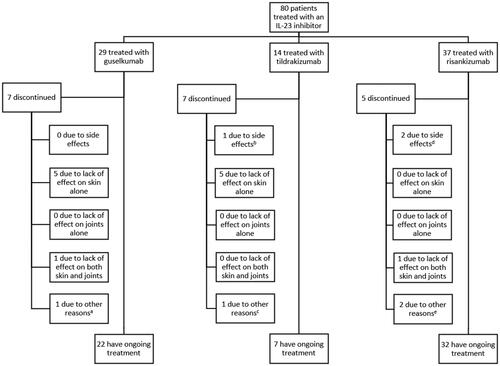
Figure 2. Kaplan–Meier estimates of (A) overall drug survival and (B) drug survival for each IL-23 Inhibitor. Patients who were still receiving treatment on the last day of follow-up were censored (illustrated with a tick mark). aThe curve for risankizumab drops to 0% survival at 69 weeks. At 69 weeks, all but one patient had either been censored or stopped treatment. The remaining patient discontinued treatment after 69 weeks. Risankizumab is the newest of the drugs, and therefore many patients in this group have been censored.
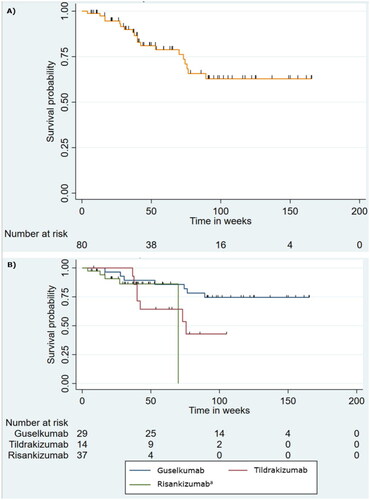
Table 2. Effectiveness overall and for each drug at the end of follow-up and weeks 12–17 and 40–60a.
