Figures & data
Figure 1. CONSORT diagram. Flowchart demonstrating patient disposition in Treatment Period 1 (Weeks 0–12) and Treatment Period 2 (Weeks 12–24). Overall, 48/52 (92.3%) completed Treatment Period 1 and 41/52 (78.8%) entered Treatment Period 2.
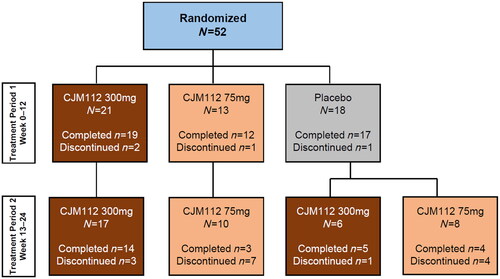
Table 1. Baseline characteristics.
Figure 2. Total inflammatory facial lesion counts up to Week 12. Line graph demonstrating the effect of CJM112 300 mg, CJM112 75 mg, and placebo on the total inflammatory facial lesion count in patients with moderate to severe inflammatory acne from baseline to Week 12. The log-transformed inflammatory facial lesion count was analyzed using a Bayesian model for repeated measurements. Data are demonstrated as geometric mean and 90% confidence intervals. Numbers, and the number of patients included in the analysis at each time point.
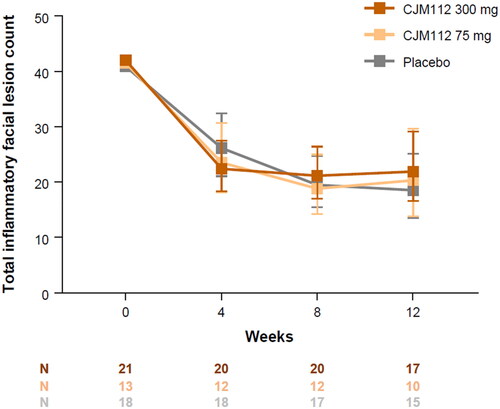
Table 2. Key efficacy endpoint results at Week 12.
Table 3. Incidence of adverse events in Treatment Period 1.
Supplemental Material
Download PDF (64.4 KB)Data availability statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
