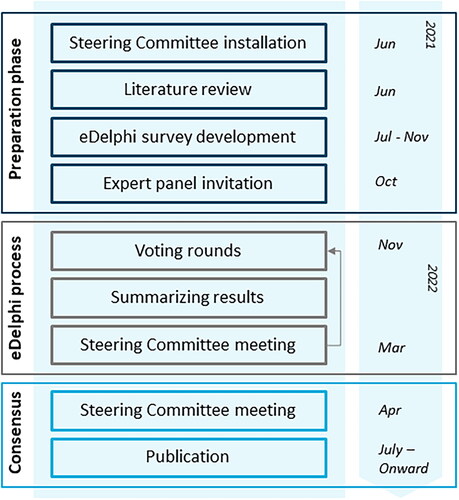
Figures & data
Table 1. Demographic characteristics of participants in the eDelphi survey.
Table 2. Results of the eDelphi consensus: criteria for biologic dose reduction in patients with psoriasis.
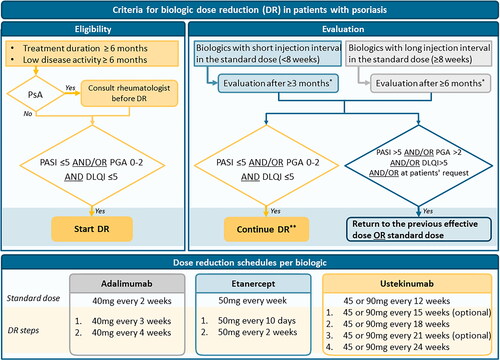
Figure 2. Algorithm for biologic dose reduction (DR) in patients with psoriasis based on the consensus. DR: dose reduction; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; PsA: psoriatic arthritis; DLQI: Dermatology Life Quality Index. *DR can be discontinued at any time point in case of increased psoriasis or at the patient’s request. **Continue DR: next step DR or continue lowered dose. For dosing schedules see the lowest part of the algorithm.
Supplemental Material
Download PDF (245.3 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.