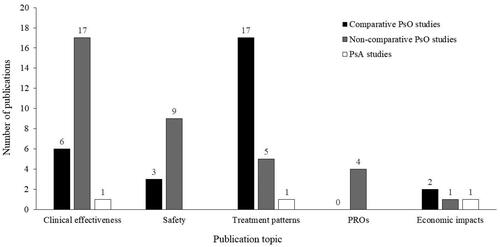
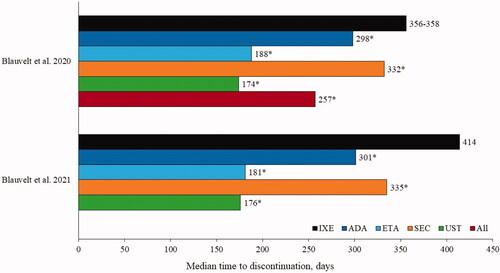
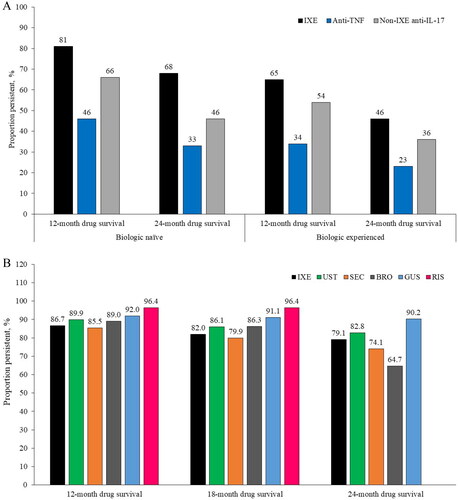
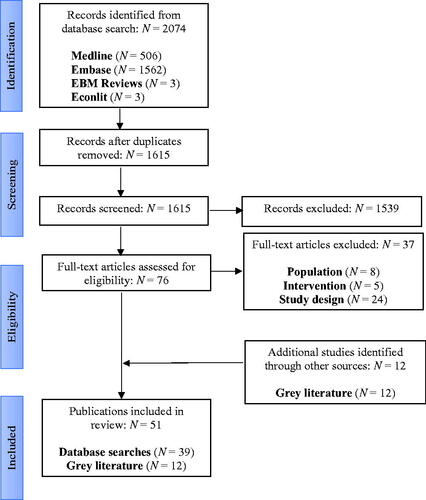
Figures & data
Table 1. Comparative studies reporting PASI75/90/100 for ixekizumab in patients with psoriasis.
Table 2. Non-comparative studies reporting PASI75/90/100 for ixekizumab.