Figures & data
Figure 1. Patient attrition flow chart. AS: ankylosing spondylitis; Ili: interleukin inhibitor; JIA: juvenile idiopathic arthritis; PSA: psoriatic arthritis; PsO: psoriasis; RA: rheumatoid arthritis; TNFi: tumor necrosis factor inhibitor; UC: ulcerative colitis.

Table 1. Baseline demographic and clinical characteristics post-propensity score matching.
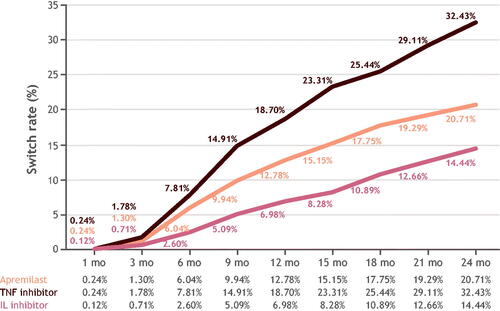
Figure 3. Kaplan–Meier curve for time to treatment modification in months, N = 6558. IL: interleukin; N/No.: number; TNF: tumor necrosis factor.
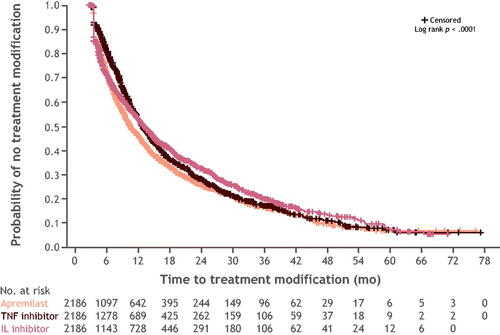
Table 2. HCRU and costs before and after switching during a 24-month follow-up perioda.
Supplemental Material
Download PDF (408.2 KB)Data availability statement
Data for these analyses were made available to the authors through third-party license from Optum Clinformatics® Data Mart, a commercial data provider in the United States. As such, the authors cannot make these data publicly available due to a data use agreement. Other researchers can access these data by purchasing a license through Optum Clinformatics® Data Mart. Inclusion criteria specified in the Methods section would allow other researchers to identify the same cohort of patients used for these analyses. Interested parties may see https://www.optum.com/business/life-sciences/real-world-data/claims-data.html for more information on Optum Clinformatics® Data Mart.