Figures & data
Table 1. Baseline demographic and clinical characteristics in the modified intention-to-treat population, and by respondera subgroups in the nemolizumab treatment group.
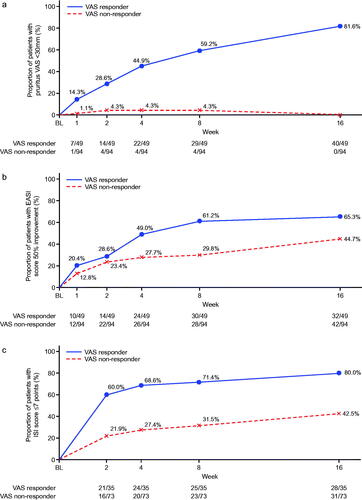
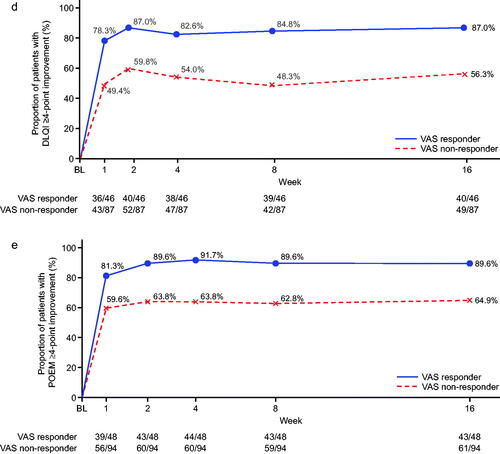
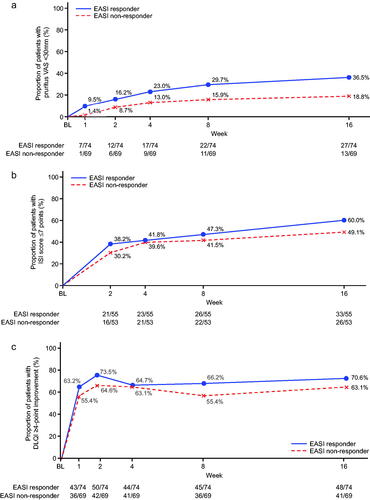
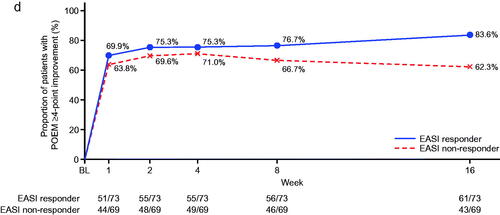
Figure 1. Subgroup analysis (analysis of covariance) for percent change from baseline at week 16 in pruritus visual analog scale (VAS) and Eczema Area and Severity Index (EASI) responders (nemolizumab treatment group). *Responder was defined as those who achieved an improvement of ≥50% from baseline at week 16 for either VAS or EASI. CI: confidence interval; DLQI: Dermatology Life Quality Index; NRS: numerical rating scale; POEM: Patient-Oriented Eczema Measure; sIGA: static Investigator’s Global Assessment.
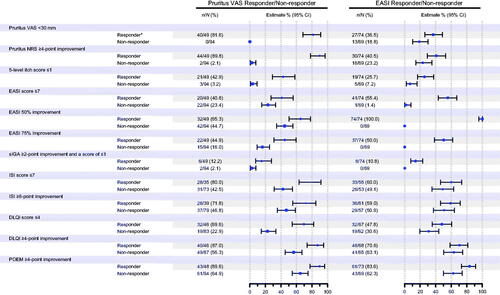
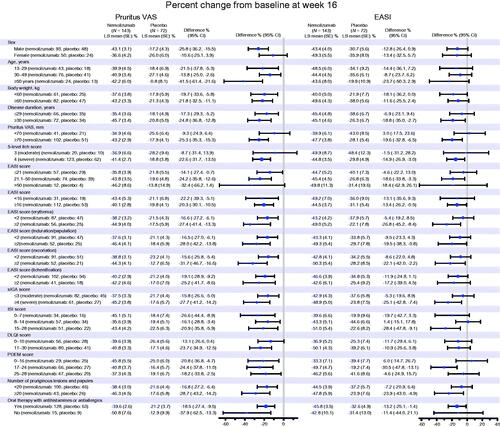
Figure 4. Subgroup analysis (analysis of covariance) for percent change from baseline at week 16 in pruritus visual analog scale (VAS) and Eczema Area and Severity Index (EASI) scores (modified intention-to-treat population). CI: confidence interval; DLQI: Dermatology Life Quality Index; ISI: Insomnia Severity Index; LS: least squares; POEM: Patient-Oriented Eczema Measure; SE: standard error; sIGA: static Investigator’s Global Assessment.
Data availability statement
The authors are unable to provide individual patient data as consent for distribution of personal information was not obtained in the clinical trial.