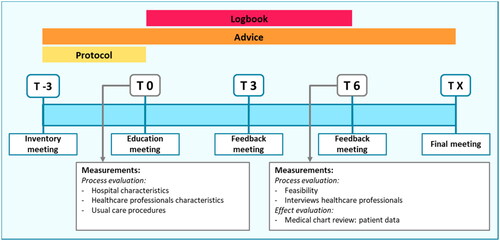
Figures & data
Figure 1. Dose reduction protocol.
Abbreviations: PASI: Psoriasis Area and Severity Index; DLQI: Dermatology Life Quality Index.
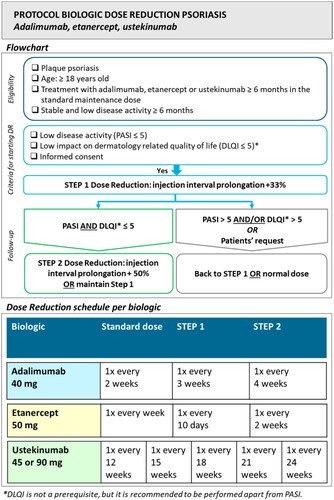
Table 1. Baseline characteristics of participating hospitals and usual care procedures.
Table 2. Summarized characteristics of involved healthcare providers.
Table 3. Overview of (sub)themes and corresponding quotes resulting from the interviews.
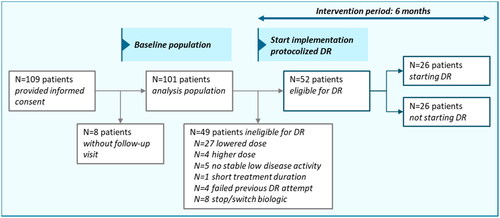
Figure 3. Flow chart of included patients, patients eligible for DR and patients starting DR during the intervention period.
Patient data were collected in 2 hospitals. Abbreviations: DR: dose reduction. DR eligibility was based on the following criteria: plaque psoriasis, sustained low disease activity ≥6 months, use of the standard maintenance dose ≥6 months, low impact of psoriasis on patients’ dermatology-related quality of life, no failed previous DR attempt.
Supplemental Material
Download PDF (321.5 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.