Figures & data
Figure 1. Study design diagram for patients who entered BREEZE-AD3 as responders or partial responders. BARI: baricitinib; TCS: topical corticosteroids. aBackground TCS may have been initiated or reinitiated at any time during the study and were to be provided as part of rescue or retreatment any time patient’s IGA score became ≥3. bEligible patients were re-randomized in the withdrawal and down-titration sub-study. Patients who did not enroll in the sub-study remained on their treatment. cPatients enrolled in the sub-study were automatically retreated if their IGA score became ≥3. dA post-treatment follow-up visit was conducted approximately 28 days after the last dose of the investigational product, either at the end of the study or following early termination.
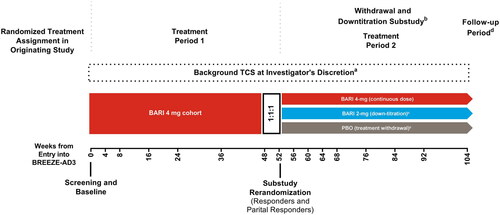
Table 1. Summary of demographics and disease characteristics at baseline at week 52 in randomized patients with atopic dermatitis treated with baricitinib continuation and down-titration substudy.
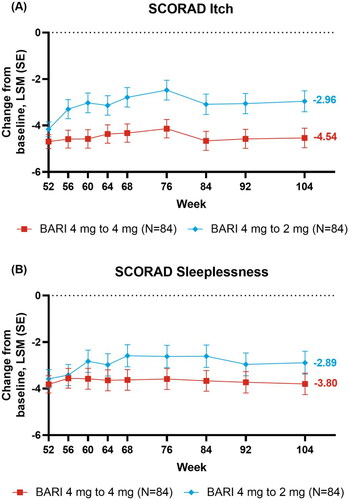
Figure 2. Skin response over time: (A) vIGA-AD (0,1), (B) EASI75, and changes from baseline over time for (C) EASI. *p ≤ .05, **p ≤ .01, and ***p ≤ .001 denote significant differences between treatment groups
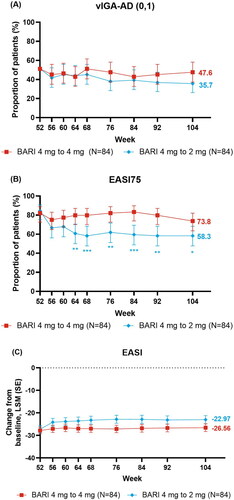
Figure 3. Patient-reported responses over time: (A) DLQI (0,1), (B) POEM ≥4 point improvement, (C) HADS-A < 8, and (D) HADS-D < 8. aIn patients with borderline or abnormal severity scores at baseline (HADS-A ≥8 or HADS-D ≥8)
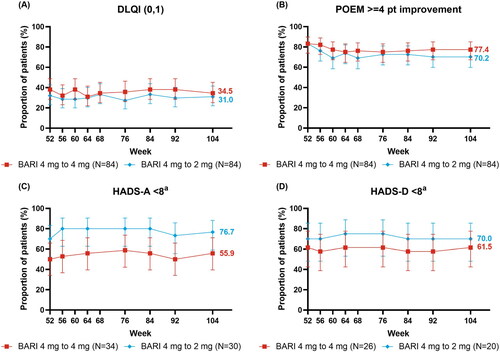
Figure 4. Changes from baseline over time 4-mg: (A) Presenteeism, (B) Absenteeism, (C) Overall Work Impairment, (D) Daily Activity§. aEmployed patients only, bEmployed for pay patients only, §Nx is the number of patients with presenteeism, absenteeism, daily activity impairment, and overall work impairment evaluated at week 52.
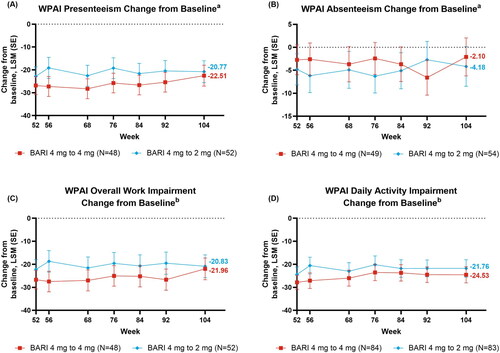
Table 2. Outcomes for physician- and patient rated assessments at week 52, 68, and 104.
Supplemental Material
Download PDF (60.4 KB)Data availability statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.