Figures & data
Table 1. Patient demographics and baseline characteristics.
Figure 1. Probability of protocol-defined flare for adolescent and adult patients during the randomized maintenance period of the JADE REGIMEN study. Flare was defined in the protocol as ≥50% loss of initial EASI response at week 12 with a new IGA score ≥2. Patients at risk were defined as patients who did not experience flare and were continuing treatment. Missing event times were considered as right censored (censored at random) on the last date of randomized treatment. Patients included in the adolescent group were aged 12–17 years. Patients included in the adult group were aged ≥18 years. CI: confidence interval; EASI: Eczema Area and Severity Index; HR: hazard ratio; IGA: Investigator’s Global Assessment.
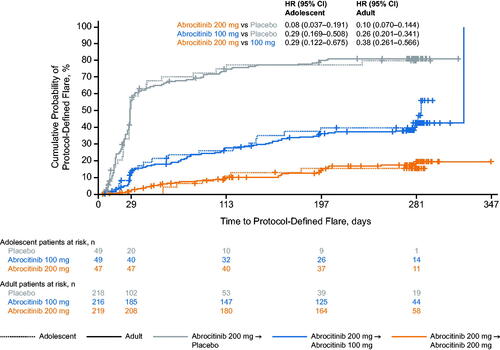
Figure 2. Proportion of patients who achieved ≥4-point improvement from study baseline in health-related quality-of-life index score at week 40 of the randomized maintenance period of the JADE REGIMEN study. Quality of life of adolescents (12–17 years old) was assessed using the CDLQI. Quality of life of adult patients (≥18 years old) was assessed using the DLQI. Study baseline was defined as the last measurement collected on or before day 1 of treatment. Patients who withdrew from the trial or experienced flare and received rescue treatment after randomization were counted as not having achieved the outcome after withdrawal. CDLQI: Children’s Dermatology Life Quality Index; CI: confidence interval; DLQI: Dermatology Life Quality Index.
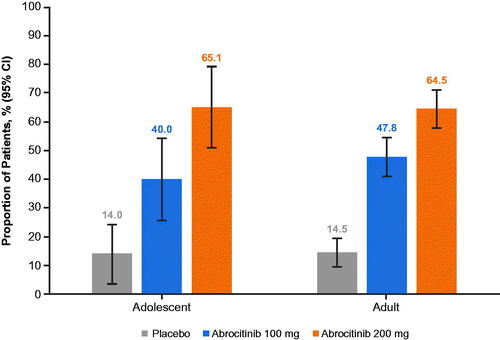
Table 2. Summary of patient-year and incidence rates for TEAEs (any causality) for adolescents and adults treated in the randomized maintenance period of JADE REGIMEN.
Supplemental Material
Download PDF (476.7 KB)Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
