Figures & data
Table 1. Baseline demographics and characteristics at MOA Level.
Figure 2. Proportion of patients with ≥2 escalated dosing intervals above the 30% threshold during the maintenance period by (A) MOA and (B) Biologic. **p < 0.01, ***p < 0.0001 compared to risankizumab based on chi-square test. IL: interleukin; MOA: mechanism of action; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
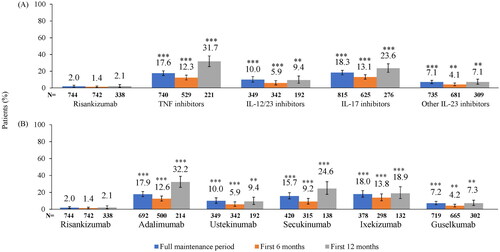
Figure 3. Adjusted odds ratios of dose escalation at the 30% and 20% thresholds by MOA. All p < 0.0001. CI: confidence interval; IL: interleukin; MOA: mechanism of action; OR: odds ratio; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
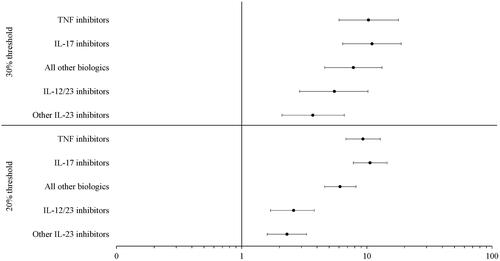
Figure 4. Adjusted odds ratios of dose escalation at the 30% and 20% thresholds by biologic. All p < 0.0001. CI: confidence interval; IL: interleukin; OR: odds ratio.
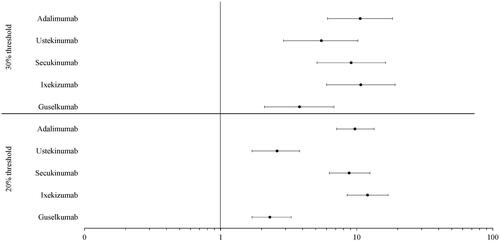
Figure 5. Proportion of patients with ≥2 escalated dosing intervals above the 20% threshold during the maintenance period by (A) MOA and (B) biologic. *p < 0.05, **p < 0.01, ***p < 0.0001 compared to risankizumab based on chi-square test. IL: interleukin; MOA: mechanism of action; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
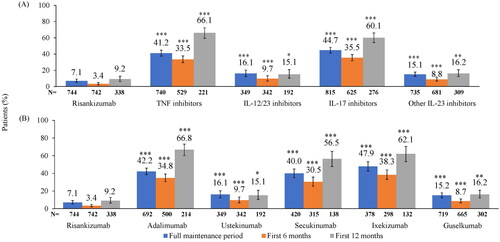
Figure A1. Patient attrition. AS: ankylosing spondylitis; CD: Crohn’s disease; HS: hidradenitis suppurativa; PsA: psoriatic arthritis; PsO: psoriatic arthritis; RA: rheumatoid arthritis; UC: ulcerative colitis; UV: uveitis. aThe date of the first biologic claim was considered the induction period index date and the type of the biologics was defined as the index biologic drug; patients with evidence of difference types of biologics use on the induction period index date were excluded. bThe start of the maintenance period was from the induction period index date + induction period + 7 grace days. cThe setting (inpatient/outpatient) of the diagnosis claim does not matter. d6 months prior to the induction period index date including the induction period index date. ePatients had no prior claim of the index biologic in the period starting from the earliest available data to the induction index date.
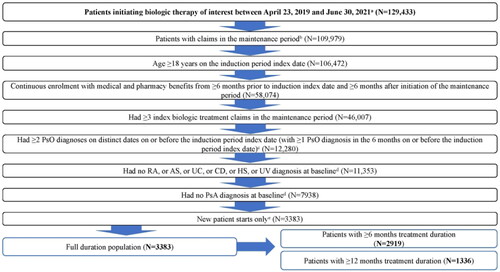
Table A1. Expected daily dosing in maintenance period.
Table A2. Baseline demographics and characteristics at biologic level.
Table A3. Average number of dose escalated claims and dose magnitude above the 30% threshold during the maintenance period by biologic.
Data availability statement
The data that support the findings of this study are available from Merative® MarketScan® Research. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author upon request with the permission from Merative® MarketScan® Research.