Figures & data
Table 1. Participant demographic characteristics overall and by AD severity.
Table 2. Quality of life in patients with ADTable Footnotea.
Table 3. Work impact of ADTable Footnotea.
Figure 1. Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitisa. Recall period: past seven days. aScores from the PO-SCORAD rounded to the first integer were used to define severity; scores ≤27 indicate mild AD, scores ≥28 to ≤56 indicate moderate AD, and scores ≥57 indicate severe AD. Four participants were missing severity level categorization due to at least one missing item on the PO-SCORAD. Employed: n = 126. For absenteeism, presenteeism, and work productivity loss scores: total, n = 126; mild, n = 33; moderate, n = 59; severe, n = 32. For activity impairment score: total, n = 186; mild, n = 50; moderate, n = 83; severe, n = 49. AD: atopic dermatitis; PO-SCORAD: Patient-Oriented SCORing Atopic Dermatitis.
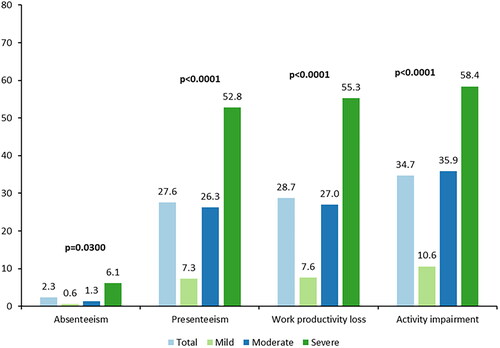
Figure 2. Treatment Satisfaction Questionnaire Medication (TSQM Version 2.0), topical and systemic medications, overall and by disease severitya,b,c. (A, B) TSQM scores for topical and systemic medications, respectively. (A) Pairwise comparisons: global satisfaction – mild vs. severe (p < .01), effectiveness – mild vs. severe (p < .001) and moderate vs. severe (p < .01), convenience – mild vs. moderate (p < .01), and mild vs. severe (p < .05). (B) Pairwise comparisons: global satisfaction – mild vs. severe (p < .01), effectiveness – mild vs. moderate (p < .01), and mild vs. severe (p < .001). aTopical medications included: corticosteroid topical cream or ointment, topical calcineurin inhibitor, topical phosphodiesterase 4 inhibitor, antihistamine by topical cream or ointment, emollient or moisturizer, itch cream, gel or ointment, and cleansers. bSystemic medications included: corticosteroid by mouth or injection, immunosuppressant, biologics (dupilumab), antihistamine by mouth, anti-microbial medication, and anti-viral medication. cScores from the PO-SCORAD were used to define severity; scores ≤27 indicate mild AD, scores ≥28 to ≤56 indicate moderate AD, and scores ≥57 indicate severe AD.
Three and two participants for topical and systemic medications respectively are missing severity level categorization due to at least one missing item on the PO-SCORAD. Missing values were not included in %.In topical medications, for global satisfaction, effectiveness score, and convenience score: total, n = 171; mild, n = 44; moderate, n = 79; severe, n = 45. For side effects score: total, n = 40; mild, n = 5; moderate, n = 19; severe, n = 16. In systemic medications, for global satisfaction, effectiveness score, and convenience score: total, n = 113; mild, n = 20; moderate, n = 55; severe, n = 36. For side effects score: total, n = 40; mild, n = 5; moderate, n = 21; severe, n = 14. AD: atopic dermatitis; PO-SCORAD: Patient-Oriented SCORing Atopic Dermatitis; TSQM: Treatment Satisfaction Questionnaire Medication.
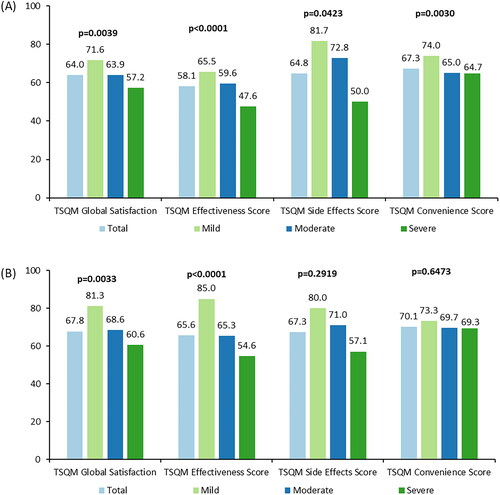
Table 4. Healthcare provider visits for atopic dermatitis treatmentTable Footnotea.
Table 5. Atopic dermatitis treatmentTable Footnotea.
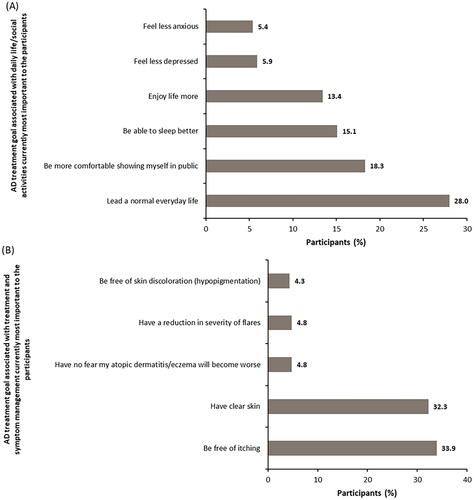
Figure 3. Atopic dermatitis treatment goal associated with daily life/social activities and treatment and symptom management currently most important to participants. (A) AD treatment goals associated with daily life/social activities currently most important to the participants. (B) AD treatment goals associated with treatment and symptom management currently most important to the participants. In figure 3A, n = 186. Other reasons accounting to <5% each were: be comfortable in my sex life (4.3%), be more productive in my everyday life (3.8%), be less of a burden on relatives/friends (1.1%), engage in normal leisure activities (1.1%), be less of a burden in my marriage or partnership (1.1%), be more productive in my working life (0.5%), and have more social contact with people (0.5%).
In figure 3B, n = 186. Other reasons accounting to <4% each were: be free of skin pain (3.2%), have a reduction in frequency of flares (3.2%), have improved predictability of atopic flares from day to day (2.7%), find a clear diagnosis and treatment plan (2.7%), have a treatment that works quickly (2.2%), be less dependent on doctor and clinic visits (1.6%), have fewer of side effects of treatment (1.6%), be free of burning sensation on skin (0.5%), spend less time on treatment (0.5%), use fewer treatments (0.5%), have fewer out-of-pocket treatment expenses (0.5%), and have confidence that therapy will work (0.5%).AD: atopic dermatitis.
Data availability statement
YouGov provided Evidera with de-identified, fully documented datasets. Evidera may provide the datasets generated during and/or analyzed during the current study upon request; contact Elizabeth Bacci at [email protected].
