Figures & data
Table 1. Demographics and baseline characteristics of patients with atopic dermatitis.
Table 2. Correlation between baseline background factors and eczema area and severity index for each anatomical site.
Figure 1. The transition of total eczema area and severity index (EASI) and EASIs of individual sites (a), medians of percent reduction of EASI (b), achievement rates of EASI 75 (c), EASI 90 (d), and EASI 100 (e) at weeks 0, 4, 12, or 24 of treatment with upadacitinib plus topical corticosteroids in patients with atopic dermatitis (n = 65). *p < .05 between trunk versus lower limbs and †p < .05 between head and neck versus lower limbs, analyzed by Friedman’s test in (b) or by Fisher’s exact test in (c, d).
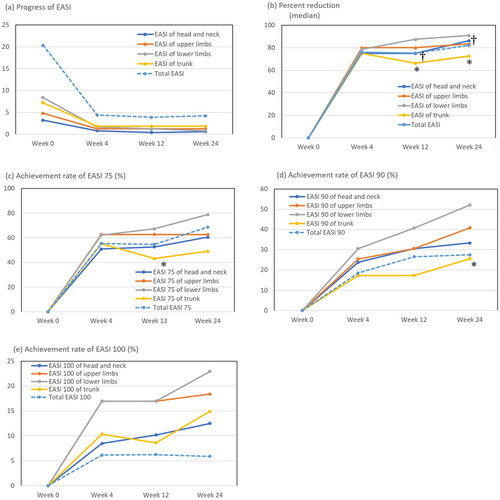
Table 3. The relations of patients’ background factors with percent reduction of EASI of head of and neck at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 4. The relations of patients’ background factors with percent reduction of EASI of upper limbs at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 5. The relations of patients’ background factors with percent reduction of EASI of lower limbs at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 6. The relations of patients’ background factors with percent reduction of EASI of trunk at weeks 4, 12, or 24 of upadacitinib treatment for patients with atopic dermatitis (n = 65).
Table 7. The predictive factors for the percent reduction of eczema area and severity index (EASI) of lower limbs at weeks 4, 12, or 24 of upadacitinib treatment assessed by linear multivariate regression analysis in patients with atopic dermatitis (n = 65).
Supplemental Material
Download Zip (58.5 KB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
