Figures & data
Table 1. Baseline characteristics of PsO/PsA patients initiating biologics or methotrexate treatment.
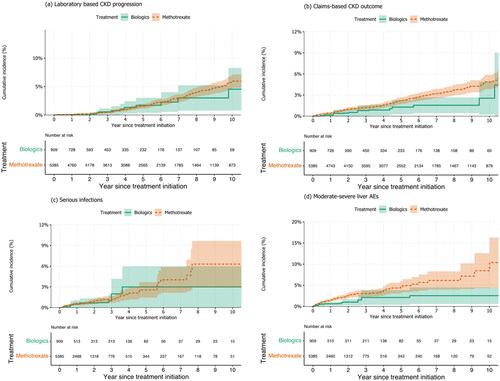
Table 2. Follow-up time, number of events, incidence rates and relative risk for the association between biologics vs. methotrexate and study outcomes.
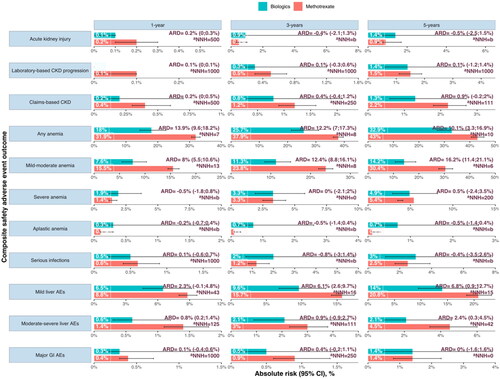
Figure 1. Absolute Risk, absolute risk difference (ARD), and estimated number needed to harm (NNH) for each composite adverse event safety outcome in PsO/PsA patient initiating methotrexate and biologics at 1, 3, and 5 years of therapy. The Nelson- Aalen estimator derived absolute risk estimates from cumulative incidence curves. Risk differences are shown per 1000 persons and rounded to the nearest integer.
aNNH can be interpreted as the number of subjects that would be required to start methotrexate (and not biologics) to observe 1 excess adverse event.
Abbreviations: CKD: chronic kidney disease; ARD: absolute risk difference; NNH: number needed to harm; b) Indicates that a negative number is needed to harm, arising when methotrexate has lower adverse event risks than biologics.
Analyses were adjusted for: age, sex, eGFR, psoriasis type, hypertension, diabetes, myocardial infarction, heart failure, atrial fibrillation, liver disease, mild infections (6 months prior), moderate-severe infections (6 months prior), history of cancer, CKD diagnosis, AKI (6 months prior), topical treatment (corticosteroids, topical calcineurin inhibitors), other systemic Pso/PsA treatment (sulfasalazine, acitretin, leflunomide, apremilast, dimethyl fumarate, cyclosporine), other medications (ACEi/ARB, beta-blocker, Lipid-lowering, anti-diabetics, systemic glucocorticoids, antimicrobials, NSAID), hs-CRP, Hb, AST, ALT and year of treatment initiation.
Supplemental Material
Download PDF (1 MB)Data availability statement
The SCREAM project contains sensitive personal data that as per GDPR regulations cannot be publicly shared. We however welcome collaborative project proposals that abide to GDPR, national and institutional regulations for data sharing and data access. For enquiries, please contact [email protected].