Figures & data
Figure 1. Impact of the treatment with tralokinumab on subjective symptoms and patients’ quality of life: achievement of pp-NRS and s-NRS reduction ≥ 4 points (a) and ADCT ≤ 7 (c) at week 16. Decrease in median pp-NRS and s-NRS (b) and median ADCT (d) after 16 weeks. pp-NRS: Peak Pruritus- Numerical Rating Scale; s-NRS: Sleep-Numerical Rating Scale; ADCT: Atopic Dermatitis Control Tool.
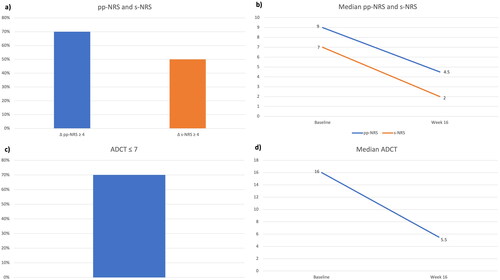
Figure 2. Clinical improvement after 16 weeks of treatment with tralokinumab: decrease in median EASI (a); percentages of patients achieving EASI 50 and EASI 75 (b), and proportion of patients reaching an IGA of 0 or 1 (c) at week 16. EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment.
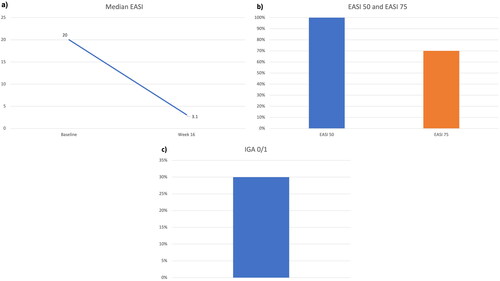
Table 1. Demographic and clinical characteristics of our patients at baseline.
Data availability statement
Additional data supporting the findings of this manuscript are available on reasonable request to the corresponding author.
