Figures & data
Table 1. Characteristics of study population (patients treated with adalimumab and methotrexate).
Table 2. Drug costs of adalimumab biosimilars, methotrexate and folic acid according to list price (in Euro).
Table 3. Retention rate at week 24 and 52 in the study population (patients treated with adalimumab and methotrexate).
Figure 1. Cost per responder analysis for patients receiving ABP 501 (Amgevita®) (black histogram), MSB11022 (Idacio®) (grey histogram) and methotrexate (white histogram) at week 24.
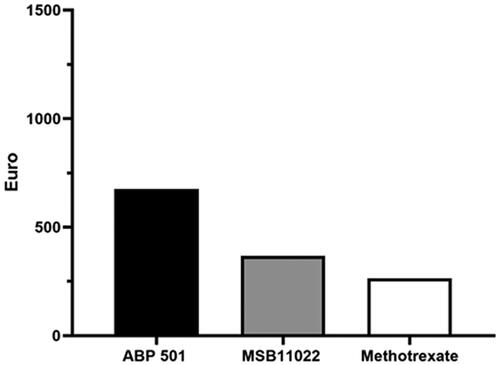
Figure 2. Cost per responder analysis for patients receiving ABP 501 (Amgevita®) (black histogram), MSB11022 (Idacio®) (grey histogram) and methotrexate (white histograms) at week 52.
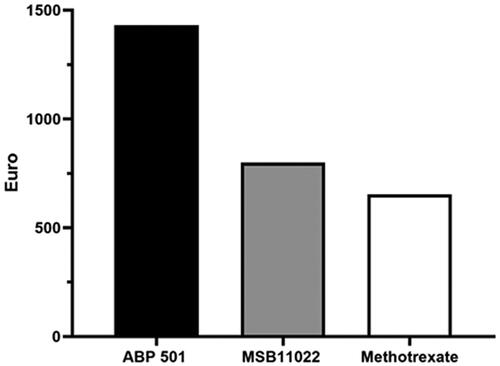
Table 4. Cost per responder of adalimumab biosimilars and methotrexate at week 24 and 52 (in Euro).
Supplemental Material
Download PDF (77.1 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
