Figures & data
Figure 1. Study designs for the phase 1 RIPT (A) and CIPT (B) studies. RIPT study: determines the potential of a topical treatment to induce sensitization (allergic potential) via repeated applications over 6–12 weeks. CIPT study: evaluates a topical treatment’s irritancy potential as a result of direct damage to the epidermal cells (without involvement of allergic or immunologic mechanism) via repeated applications over 21 days. aParticipants were only re-challenged to a study material if there were signs suggestive of contact sensitization observed at any challenge evaluations or at the investigator’s discretion; this occurred approximately 4 weeks after the completion of the Challenge phase. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; RIPT: repeat insult patch test; SLS: sodium lauryl sulfate; tiw: three times weekly.
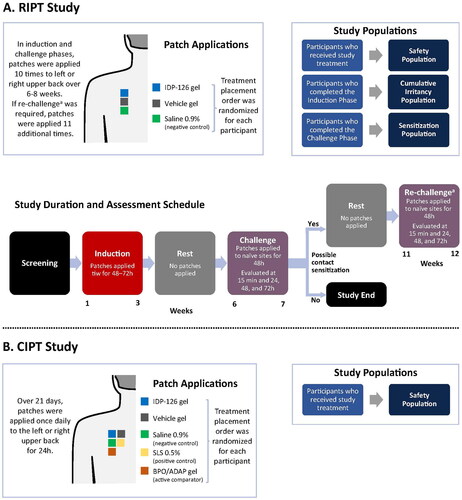
Table 1. Clinical Grading and Corresponding Numeric Scores (Phase 1 Studies).
Table 2. Participant Demographics and Baseline Characteristics (Safety Populations).
Figure 2. Mean cumulative irritation scores (RIPT cumulative irritancy population; CIPT safety population). ***p < 0.001 vs IDP-126 gel. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; RIPT: repeat insult patch test; SD: standard deviation; SLS: sodium lauryl sulfate.
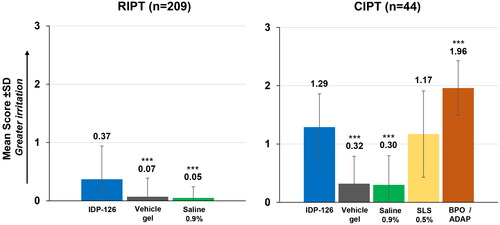
Figure 3. Normalized total irritation scores (CIPT safety population). ***p < 0.001 vs IDP-126 gel. Normalized irritation scoring: 0–49 = no significant irritation; 50–199 = slightly irritating; 200–449 = moderately irritating; 450–630 = highly irritating. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; SLS: sodium lauryl sulfate.
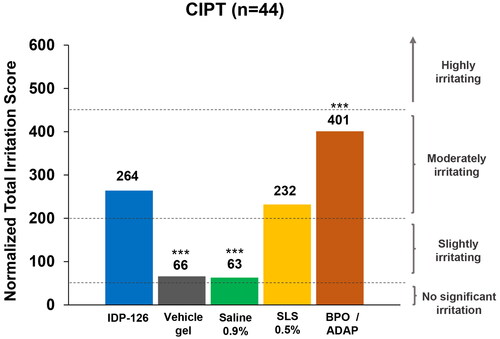
Table 3. Treatment-Emergent Adverse Events (Safety Populations).
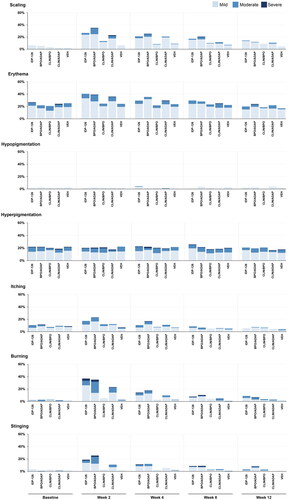
Figure 4. Cutaneous safety and tolerability by visit (Phase 2 safety population). Data for “none” are not shown. No imputation of missing data. Investigator-assessed cutaneous safety (scaling, erythema, hypopigmentation, hyperpigmentation) and participant-assessed tolerability (itching, burning, stinging) were evaluated using a 4-point scale where 0 = none and 3 = severe. ADAP: adapalene 0.15%; BPO: benzoyl peroxide 3.1%; CLIN: clindamycin phosphate 1.2%; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; VEH: vehicle gel. Baseline N values: IDP-126 gel, n = 141; BPO/ADAP gel, n = 146; CLIN/BPO gel, n = 144; CLIN/ADAP gel, n = 148; VEH gel, n = 146.
Data availability statement
Data available upon request.