Figures & data
Table 1. Baseline demographics and characteristics.
Figure 1. Conditional relative attribute importance for respondents. The conditional relative importance is the difference between the preference weights on the most influential attribute level and the least influential attribute level. These differences are summed across attributes and the sum is scaled to 100. Vertical bars surrounding each relative importance weight estimate denote the 95% confidence interval around the point estimate (computed by the delta method). aA greater value represented a greater importance to patients, conditional on the attributes and levels included in the survey. bImprovement in itch was assessed from levels of 10% to 100%. cChance of clear or almost clear skin was assessed from levels of 5% to 70%. dTime until itch reduction was noticeable was assessed from levels of 1 to 14 days. eAnnual risk of acne due to treatment was assessed from levels of 0% to 25%. fAnnual risk of a serious infection due to treatment was assessed from levels of 0% to 2%. gNeed for topical corticosteroids was assessed from levels of ‘yes’ or ‘no’.
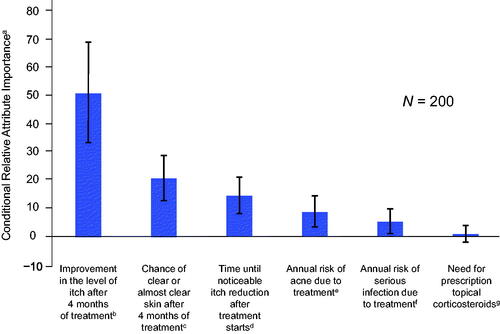
Figure 2. Attribute preference weights for respondents. The parameter estimates are the preference weights corresponding to the effects-coded attribute levels. The preference weights corresponding to the effects-coded variables are categorical variables ranging from −1 to 1. The preference weights corresponding to the effects-coded variables are log odds, which are distributed symmetrically around zero. The vertical bars surrounding each mean preference weight denote the 95% confidence interval of the point estimate (computed by the delta method for the level omitted in estimation for model specification).
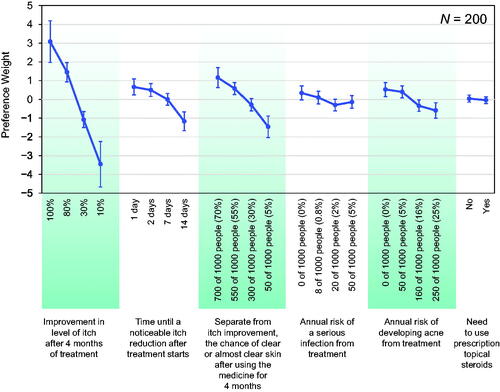
Figure 3. Minimum acceptable benefit (MAB) in improvement in level of itch after 4 months of treatment for a given change in treatment attributes (N = 200). MAB is shown as the percentage-point increase in improvement in level of itch after 4 months of treatment with 95% confidence interval. When the 95% confidence interval includes 0, the minimum acceptable benefit is not statistically different from 0. The MAB calculations hold constant the levels of all attributes other than the 1 attribute that is changing. N/A (not applicable) means that the MAB is undefined because none of the attribute levels affected treatment choice.
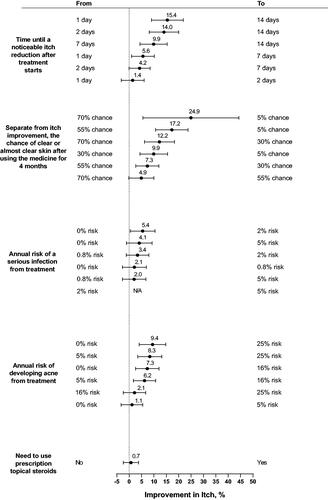
Table 2. Maximum acceptable risk of serious infection from treatment for a given change in treatment attributes (N = 200).
Supplemental Material
Download PDF (276.9 KB)Data availability statement
AbbVie is committed to responsible data sharing regarding the studies we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.
