Figures & data
Table 1. Baseline demographics and clinical characteristics.
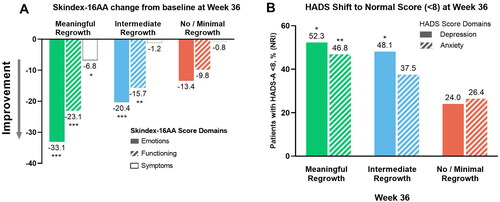
Figure 1. Least square mean change from baseline to Week 36 in Skindex-16 AA domain scores (A) and percentage of patients in whom scores shifted from B/AB to normal HADS-a and HADS-D scores at Week 36 (B). *p < 0.05; **p < 0.01; ***p < 0.001 vs no or minimal regrowth. B/AB: borderline/abnormal; HADS-A: Hospital Anxiety and Depression Scale-anxiety; HADS-D: Hospital Anxiety and Depression Scale-depression; SALT: Severity of Alopecia Tool.
Data availability statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and European Union, and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
