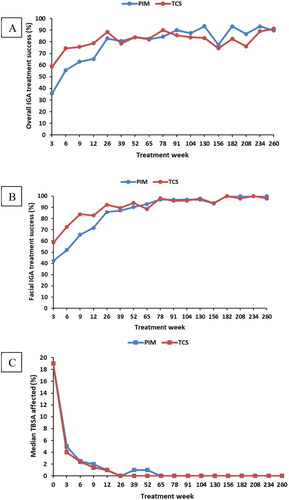
Figures & data
Figure 1. Patient disposition (TCS: Topical corticosteroid; ITT: Intent-to-treat; PIM: Pimecrolimus 1%).
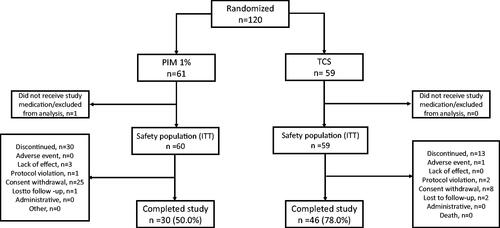
Table 1. Baseline demographics of the Chinese sub-group population (TCS: Topical corticosteroid; PIM: Pimecrolimus 1%).
Figure 2. TCS sparing effect of PIM. Graph shows number of patients in PIM group who used TCS as rescue medication.
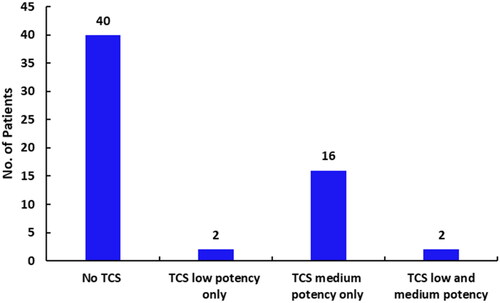
Table 2. Number of patients (%) with adverse events (≥ 30%) by preferred term and treatment group during the treatment period—Chinese sub-group population.
Data availability statement
Raw data were generated at Viatris. Derived data supporting the findings of this study are available from the corresponding author X.D. on request.