Figures & data
© 2023 eli lilly Japan K.K
Figure 1. Study design.
aFirst psoriasis diagnosis in the index period.
bDate of the first IL-17i class drug prescription after the first psoriasis diagnosis.
cPre-index period is the time from six months prior to the index date.
DB: database; IL-17i: interleukin-17 inhibitor.
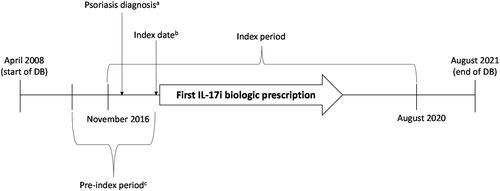
Figure 2. Selection of the study population.
Patients using SEC or BRO were excluded if the following three criteria were met – their pre-index period began before 1 November 2016; they experienced SEC or BRO between the start of the pre-index period and 1 November 2016; and they did not switch to other IL-17i drugs but continued the same treatment after 1 November 2016. Patients diagnosed with PsO were excluded if they had PsA, GPP, or EP as comorbidities within 6 months before the index date. Patients diagnosed with GPP, EP, or PsA were excluded if they had a PsO diagnosis code before diagnosis of GPP, EP, or PsA; and experienced IXE, SEC, or BRO in the pre-index period, but continued the same treatment (including generics). Patients with a diagnosis of ankylosing spondylitis (ICD-10 code M45) or non-radiographic axial spondyloarthritis (ICD-10 code M46.8) during the pre-index period were excluded.
BRO: brodalumab; EP: erythrodermic psoriasis; GPP: generalized pustular psoriasis; ICD-10: International Classification of Diseases, Tenth Revision; IL-17i: interleukin-17 inhibitors; IXE: ixekizumab; Ps: psoriasis; PsA: psoriatic arthritis; PsO: psoriasis vulgaris; SEC: secukinumab; WHO: World Health Organization.
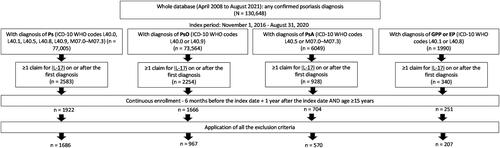
Table 1. Number of patients on IL-17 inhibitors in each cohort.
Table 2. Patient characteristics for IL-17 inhibitor class drug users.
Table 3. Treatment outcomes for IL-17 inhibitor class drug users till the end of the follow-up period.
Figure 3. IL-17i treatment persistence among patients with Ps, PsO, PsA, or GPP or EP.
Figures A-D show the Kaplan-Meier curves for persistence of IL-17i class drugs in patients with Ps, PsO, PsA, or GPP or EP, respectively.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
EP: erythrodermic psoriasis; GPP: generalized pustular psoriasis; IL-17i: interleukin-17 inhibitor; Ps: psoriasis; PsA: psoriatic arthritis; PsO: psoriasis vulgaris.
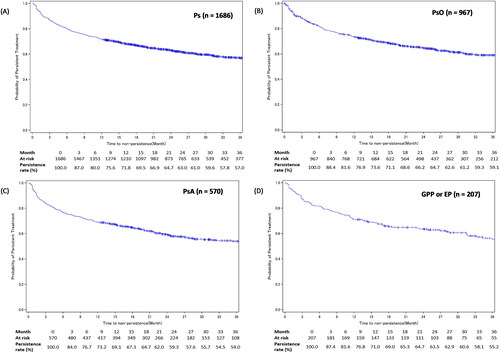
Figure 4. IL-17i treatment persistence among patients with PsO or PsA – stratified by experience with biologics.
Figures A-B show the Kaplan-Meier curves for persistence of IL-17i class drugs in bio-naïve and bio-experienced patients with PsO. Figures C-D show the Kaplan-Meier curves for persistence of IL-17i class drugs in bio-naïve and bio-experienced patients with PsA.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
IL-17i: interleukin-17 inhibitor; PsA: psoriatic arthritis; PsO: psoriasis vulgaris.
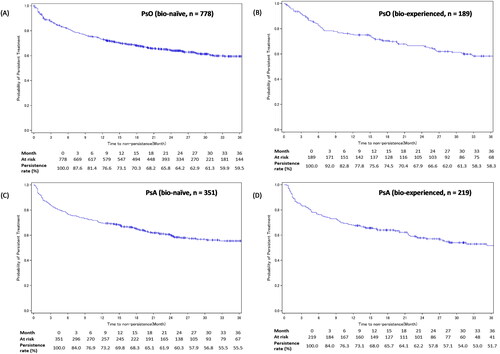
Table 4. Patient characteristics of PsO-diagnosed IXE, SEC, or BRO users.
Table 5. Treatment outcomes for PsO-diagnosed IXE, SEC, or BRO users.
Figure 5. Treatment persistence of IXE, SEC, or BRO in patients with PsO – stratified by experience with biologics.
Figure A shows the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in patients with PsO. Figures B and C show the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in bio-naïve and bio-experienced patients with PsO.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
BRO: brodalumab; IXE: ixekizumab; PsO: psoriasis vulgaris; SEC: secukinumab.
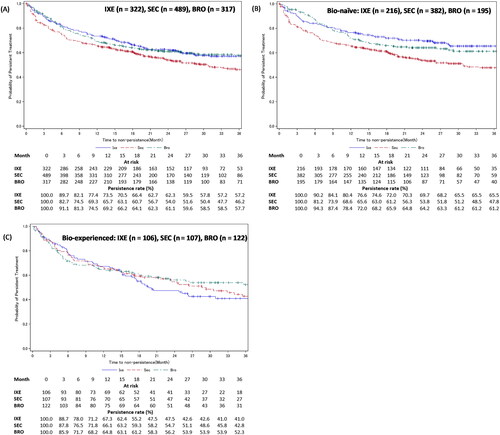
Table 6. Patient characteristics of PsA-diagnosed IXE, SEC, or BRO users.
Table 7. Treatment outcomes for PsA-diagnosed IXE, SEC, or BRO users.
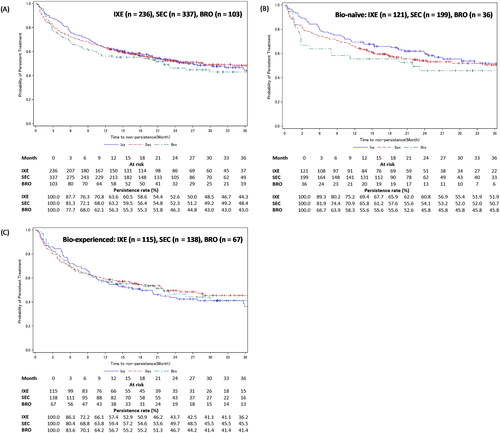
Figure 6. Treatment persistence of IXE, SEC, or BRO in patients with PsA – stratified by experience with biologics.
Figure A shows the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in patients with PsA. Figures B and C show the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in bio-naïve and bio-experienced patients with PsA.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
BRO: brodalumab; IXE: ixekizumab; PsA: psoriatic arthritis; SEC: secukinumab.
Supplemental Material
Download PDF (89.1 KB)Data availability statement
The datasets generated and/or analyzed in the current study are not publicly available because they were commercially obtained from Medical Data Vision Co., Ltd., and were used under license. Data can be made available from Medical Data Vision Co., Ltd., upon request.
