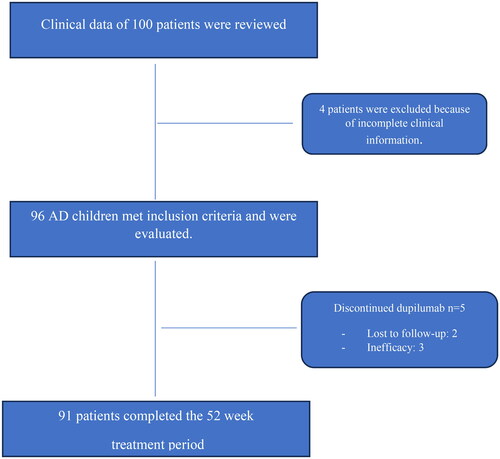
Figures & data
Table 1. Outcome of therapy with dupilumab at week 16, week 24 and week 52.
Table 2. Demographic and clinical characteristics of the patient population (n =96).
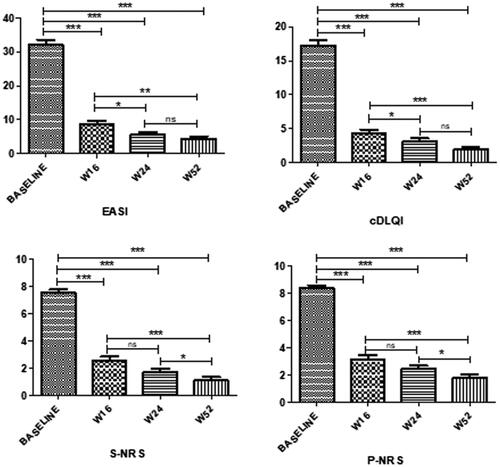
Figure 2. Outcome at week 16, week 24, and week 52 of therapy with dupilumab in 91 Childrem 6-11 years of age with moderate-to-severe atopic dermatitis.
EASI: Eczema Area and Severity Index; S-NRS: Sleep Numerical Rating Scale; P-NRS: Pruritus Numerical Rating Scale; c-DLQI: Children’s Dermatology Life Quality Index; W: week of dupilumab treatment; ***p < 0.0001; **p < 0.001; *p < 0.005; ns: not significant.
Data availability statement
Data are reported in the current study.