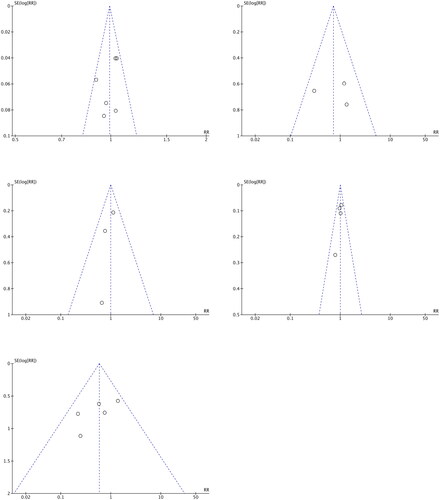
Figures & data
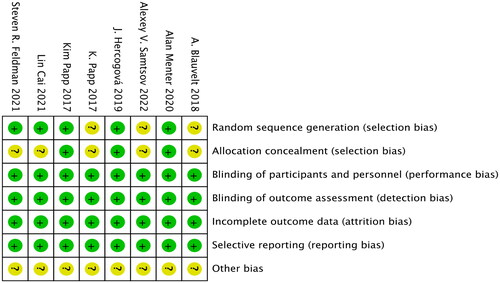
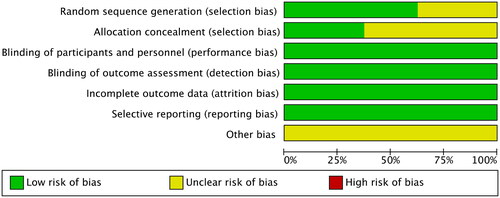
Table 1. Quality assessment of the included studies.
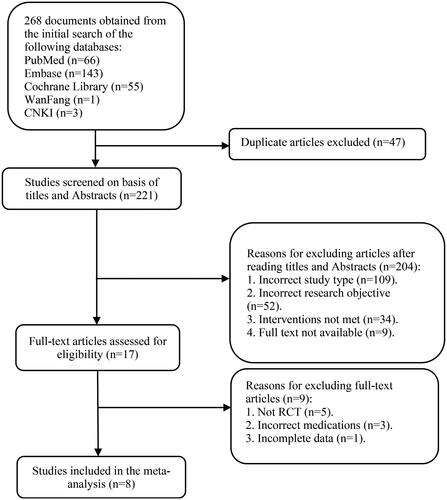
Table 2. Characteristics of included studies.
Figure 4. Forest plots of PASI 50, PASI 75, PASI 90 and PASI 100 values for adalimumab use in patients with psoriasis.
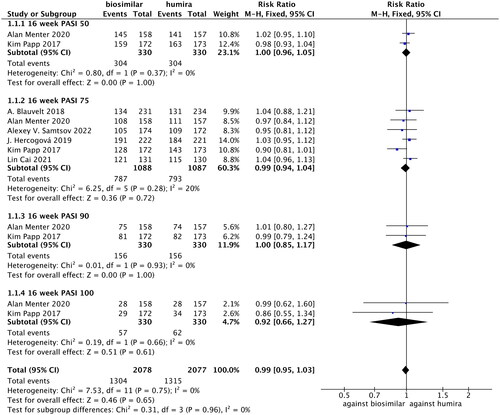
Figure 5. Forest plots of safety indicators for 16 weeks of adalimumab use in patients with psoriasis.
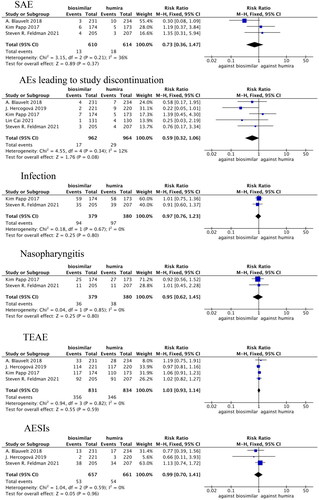
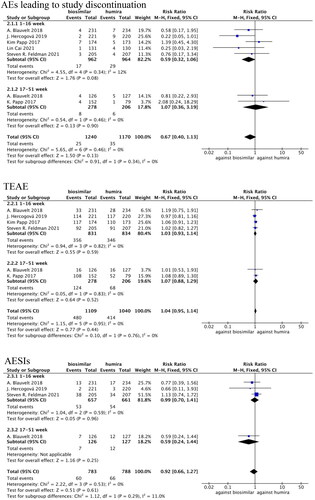
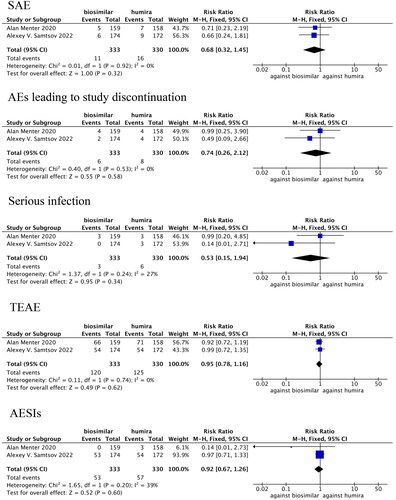
Figure 6. Forest plots of safety indicators for 1–24 weeks of adalimumab use in patients with psoriasis.
Data availability statement
The data that support the findings of this study are stored in the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China), and available from the corresponding author on reasonable request.