Figures & data
Figure 1. A simplified overview of the presumed pathomechanism and mode of action of drugs approved for HAE-1 and HAE-2, acute and prophylactic treatment. The drugs are marked in yellow, pink lightnings mark known mutations leading to HAE-nC1-INH.
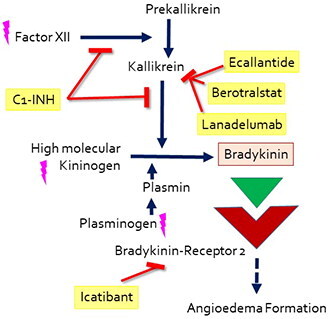
Figure 2. The highly significant negative correlation between the year of birth and the age of initial diagnosis (Spearman correlation; p = <.0001).
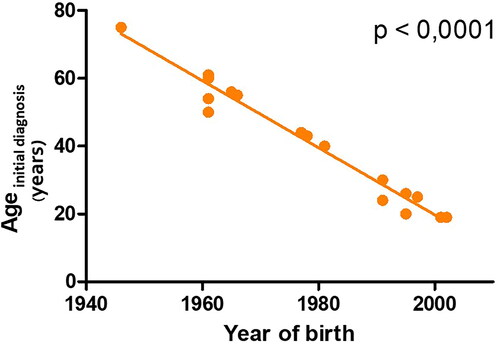
Table 1. All study patients (n = 18) with HAE-nC1-INH and the family members with a history of angioedema, the mutations and clinical information are listed.
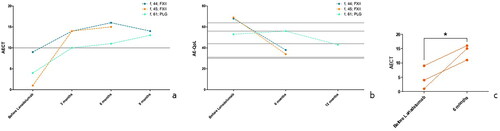
Figure 3. (a) The course of all three patients with HAE-nC1-INH (factor XII: n = 2, PLG: n = 1) under long-term prophylaxis with lanadelumab 300 mg SC measured with AECT (Angioedema Control Test). Ten and more points in AECT reflect a sufficiently controlled disease situation. (b) The course of all three patients with HAE-nC1-INH (Factor XII: n = 2, PLG: n = 1) under long-term prophylaxis with lanadelumab 300 mg SC measured with AE-QoL (Angioedema Quality of Life Questionnaire). In AE-QoL (0–100), a high score reflects a low quality of life. (c) AECT results, before and after comparison, *= p < .05; one-tailed Kruskal–Wallis test.