Figures & data
Table 1. Baseline characteristics of all the patients included.
Table 2. Efficacy responses at week 4 after single-dose treatment.
Figure 1. Proportion of patients achieving PASI 75 at week 4 in groups with or without metabolic syndrome. With metabolic syndrome vs. metabolic syndrome: 5.9% vs. 36.9%, p = 0.010. PASI 75: ≥75% reduction in Psoriasis Area and Severity Index.
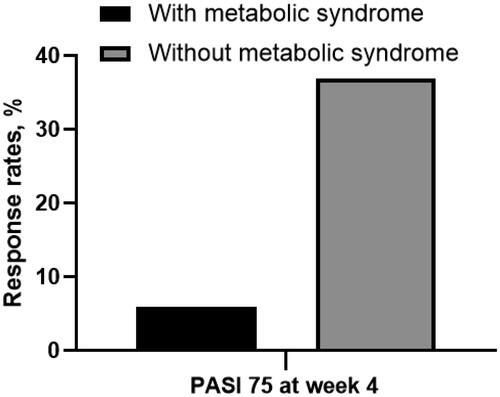
Figure 2. Proportion of patients achieving PASI 75 at week 4 in male or female patients. Male vs. female: 10.5% vs. 26.3%, p = 0.047. (PASI 100: 100% reduction in Psoriasis Area and Severity Index).
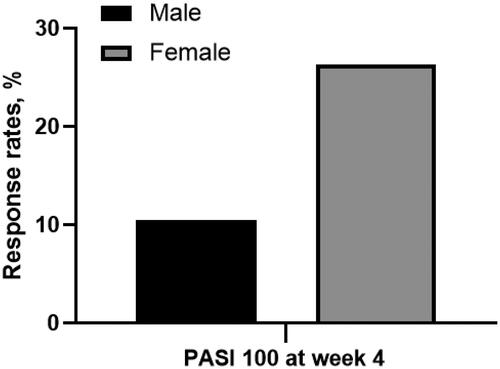
Figure 3. Proportion of patients achieving PASI 75 at week 4 in groups with or without family history of psoriasis. With family history vs. without family history: 44.4% vs. 13.9%, p = 0.044. (PASI 100: 100% reduction in Psoriasis Area and Severity Index).
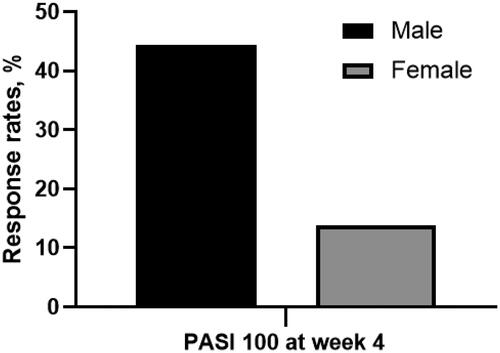
Table 3. Results of difference analysis between groups whether achieving PASI 75/90/100 at week 4.
Table 4. Association between PASI 75 /90/100 response and baseline characteristics.
Table 5. Adverse events during the 4-week treatment period.
Data availability Statement
All data generated or analyzed during this research are included in this article. Further requires can be directed to the corresponding authors.
