Figures & data
Table 1. Baseline demographics and disease characteristics.
Figure 1. Proportion of patients with and without itch improvement (responders and non-responders) achieving each DLQI endpoint after 16 weeks overall or combined lebrikizumab 250 mg and placebo (panels a and b); treated with lebrikizumab 250 mg (panels c and d); and treated with placebo (panels e and f) in ADvocate1 and ADvocate2, respectively. Abbreviations: DLQI: Dermatology Life Quality Index; R: Responder; Non-R: Non-responder; NRI: non-responder imputation; NRS: Numeric Rating Scale. Note. An itch responder (itch improvement) is defined as reporting ≥4-point reduction in Pruritus NRS scores from baseline to Week 16.
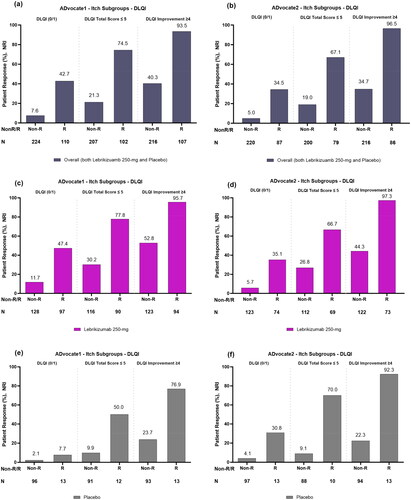
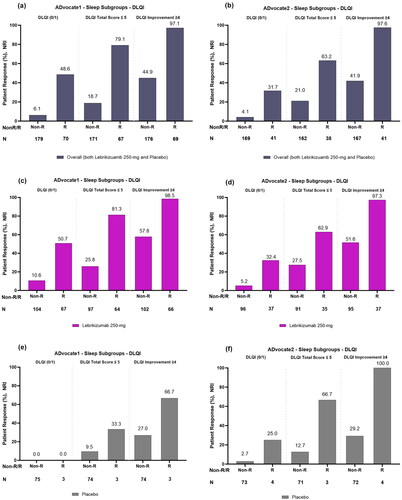
Figure 2. Proportion of patients with and without sleep improvement (responders and non-responders) achieving each DLQI endpoint after 16 weeks overall or combined lebrikizumab 250 mg and placebo (panels a and b); treated with lebrikizumab 250 mg (panels c and d) and treated with placebo (panels e and f) in ADvocate1 and ADvocate2, respectively. Abbreviations: DLQI: Dermatology Life Quality Index; R: Responder; Non-R: Non-responder; NRI: non-responder imputation. Note: A Sleep-Loss Scale responder (sleep improvement) is defined as reporting a Sleep-Loss Scale ≥2 point reduction from baseline to Week 16. Sleep improvement is improvement of itch interference on sleep.
Data availability statement
Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank, or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
